Antimicrobial susceptibility data of S. sonnei in France
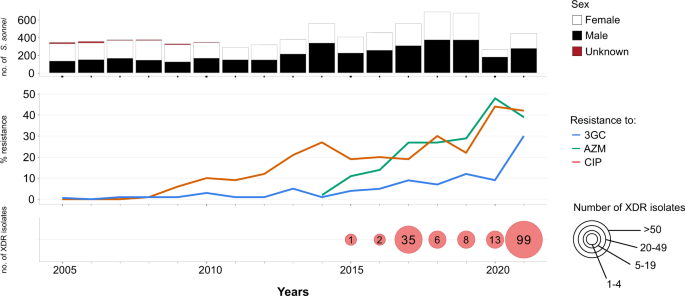
Our review of S. sonnei antimicrobial susceptibility data obtained between 2005 and 2021 (based on 7121 isolates received and confirmed at the French National Reference Center for E. coli, Shigella and Salmonella, FNRC-ESS, Institut Pasteur) revealed a sharp increase in the percentage of isolates resistant to 3GCs, CIP and AZM (P < 0.001) (Fig. 1). A first isolate resistant to 3GCs was identified as early as 2005 (prevalence of 1/342, 0.3%), whereas the first isolates resistant to CIP were identified in 2008 (prevalence of 2/373, 0.5%). Ten isolates resistant to AZM were detected in 2014, the year in which screening for such resistance began (in April) (10/557, 1.8%). In 2021, 29.5% (131/444) of S. sonnei isolates were resistant to 3GCs, 42.3% (188/444) were resistant to CIP and 38.7% (172/444) were resistant to AZM (Fig. 1). The first XDR S. sonnei isolate was identified in 2015, and an additional 163 XDR isolates have since been obtained (Fig. 1 and Supplementary Tables 1 and 2). All but one of these 164 XDR isolates were collected from mainland France. The percentage of XDR S. sonnei isolates increased over the study period (P < 0.001), peaking at 22.3% (99/444) in 2021. All but one of these XDR isolates were also resistant to trimethoprim/sulfamethoxazole and 87.2% (143/164) were resistant to tetracyclines. All these isolates remained susceptible to carbapenems.
The upper panel shows the number of isolates, by patient sex, received and analyzed per year. The middle panel shows the percentage of isolates displaying phenotypic resistance to ciprofloxacin (CIP), azithromycin (AZM), or third-generation cephalosporins (3GCs). The lower panel shows the number of extensively drug-resistant (XDR) S. sonnei isolates detected per year. XDR means combined resistance to CIP, AZM, and 3GCs.
One limitation to this study is that shigellosis is not a mandatory notifiable disease in France. Thus, laboratory-based surveillance, despite its official introduction 50 years ago, captures only a portion of the shigellosis burden (see Methods). The COVID-19 pandemic also had a clear impact on our surveillance data, as the number of S. sonnei infections in France decreased sharply in 2020 (as also reported elsewhere in Europe5) and, to a lesser extent, in 2021. This decrease may reflect a true decrease in the number of cases due to travel restrictions (43 and 30% decreases in the proportion of S. sonnei cases reporting international travel in 2020 and 2021, respectively, relative to 2019, in our surveillance data), stricter hygiene measures, lockdowns and school closures. However, it may also reflect poorer access to the healthcare system for patients with mild infections. In addition, it is not possible to rule out better referral to the FNRC-ESS of unusual XDR isolates by the clinical laboratories participating in our network. The high percentage of XDR S. sonnei observed in 2021 might, therefore, constitute an overestimate. However, in 2022, the year in which most public health measures against COVID-19 were lifted in France, XDR S. sonnei still accounted for 21.7% (73/336) of all S. sonnei isolates received from January 1 to August 31 at the FNRC-ESS.
Phylogenomics of the XDR S. sonnei isolates in France
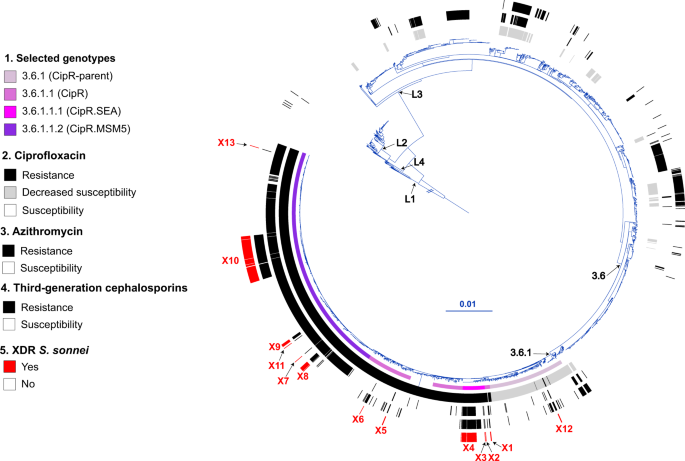
We investigated phylogenetic relationships by sequencing the genomes of the 164 French XDR S. sonnei isolates. We also included 2976 genomes from isolates and historical strains from the FNRC-ESS collected between 1945 and 2021 in the phylogenomic analysis, to provide a phylogenetic context for the XDR S. sonnei isolates. A maximum likelihood (ML) phylogenetic tree was built from an alignment of 59,295 chromosomal recombination-filtered single-nucleotide variants (SNVs) to obtain an accurate view of the evolutionary relationships (vertical evolution) between S. sonnei strains. This ML tree revealed that the 164 XDR S. sonnei isolates were not grouped into a single cluster, instead forming 13 different clusters (X1 to X13), all within lineage L3, the predominant lineage of S. sonnei worldwide over the last two decades (Fig. 2)15,16.
The S. sonnei reference genome 53G (GenBank accession numbers NC_016822) was also included in addition to the 3140 genomes from the French National Reference Center for E. coli, Shigella, and Salmonella (Supplementary Data 1). The circular phylogenetic tree (in blue) is rooted on S. flexneri 2a strain 2457T and its branch length has been shortened by a factor of 100 (indicated by the double slash) to improve vizualisation. S. sonnei lineages L1 to L4, clade 3.6, and subclade 3.6.1 are indicated. The rings show the associated information (see key) for each isolate, according to its position in the phylogeny, from the innermost to the outermost, in the following order: (1) selected S. sonnei genotypes; (2) antimicrobial susceptibility testing (AST) for ciprofloxacin; (3) AST for azithromycin; (4) AST for third-generation cephalosporins; (5) and the XDR isolates (with the XDR cluster names, X1 to X13, in red). The scale bar indicates the number of substitutions per variable site (SNVs). Due to a deliberate strategy to enrich our genomic dataset with azithromycin-resistant, ciprofloxacin-resistant, and third-generation cephalosporin-resistant isolates collected before 2017, the year in which routine genomic surveillance began in France (see “Methods”), there is an overrepresentation of these resistances in this figure with respect to the global population of 7121 isolates studied.
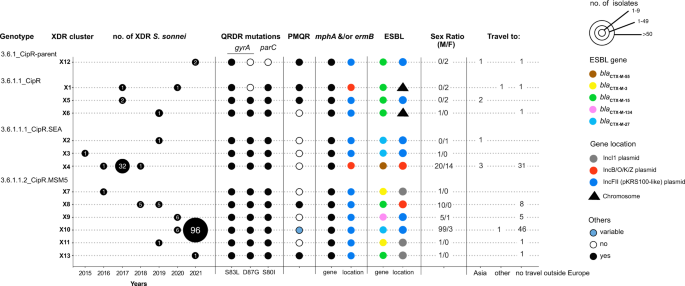
Using a new genomic tool based on a selection of core-genome SNVs developed by Hawkey et al. 15 for categorizing S. sonnei into informative phylogenetic genotypes with a universal nomenclature, we assigned the 164 XDR isolates to four different subtypes of subclade 3.6.1 (Fig. 2). The two clusters with the largest numbers of XDR isolates were X10 (n = 102) belonging to genotype 3.6.1.1.2_CipR.MSM5 (lineage L3, clade 6, subclade 1, subtype 1.2 with alias name CipR.MSM5, as used in previous publications for isolates resistant to CIP in GBMSM10,15), and X4 (n = 34) belonging to genotype 3.6.1.1.1_CipR.SEA (lineage L3, clade 6, subclade 1, subtype 1.1 with alias name CipR.SEA, as used in previous publications for isolates resistant to CIP in Southeast Asia6,15) (Fig. 3). Some of the XDR S. sonnei isolates belonging to genotypes 3.6.1_CipR-parent, 3.6.1.1_CipR and 3.6.1.1.1_CipR.SEA were acquired following travel (mostly to South and Southeast Asia) (Fig. 3). For cluster X4 (genotype 3.6.1.1.1_CipR.SEA), three patients reported travel to Southeast Asia, including one who was the index case of an outbreak at an elementary school (91 cases identified) in 2017, leading to the temporary closure of the school17. The widespread genotype 3.6.1.1.2_CipR.MSM5 was previously reported to be associated with GBMSM in the UK and Australia10,15. In our study, male patients were overrepresented (117 male and four female patients), particularly those aged 13 to 68 years (median age: 34 years; interquartile range: 28–42 years), among the cases caused by this genotype (Fig. 3). Furthermore, all but one of the cases infected with this genotype for whom travel information was available reported no travel outside Europe. Our findings indicate that the 164 XDR isolates were found in different transmission networks, with 31 cases in a school setting and eight cases in travelers returning from outside Europe. The male-to-female case ratio suggested that the majority of the other XDR cases may have been associated with GBMSM networks. However, this study was based on routine laboratory-surveillance data, with no record of the sexual orientation of the patient on the notification form accompanying the bacterial isolates sent to the FNRC-ESS (see “Methods”) and the patients were not interviewed to determine whether they were GBMSM.
The number of XDR isolates, categorized by genotype and XDR genomic cluster, is given by year of isolation. For each XDR genomic cluster, the mechanisms of resistance to CIP (mutation in the quinolone resistance-determining (QRDR) region of gyrA and parC, presence of a plasmid-mediated quinolone (PMQR) resistance gene), AZM (presence of mph(A) and/or erm(B) resistance genes) or 3GCs (presence of the extended-spectrum beta-lactamase (ESBL) blaCTX-M genes indicated in the key) are indicated. The chromosomal or plasmid location of the AZM and 3GC resistance genes is indicated. For plasmid-borne genes, the type of plasmid is also indicated. Finally, the sex ratio and information about travel (when known) are also indicated for the cases associated with each XDR genomic cluster. The two cases indicated as “Travel to other” are an individual residing in a French overseas territory in the Indian Ocean (cluster X1) and a traveler returning from Central Africa (X10).
Analysis of the AMR genes and structures in XDR S. sonnei isolates
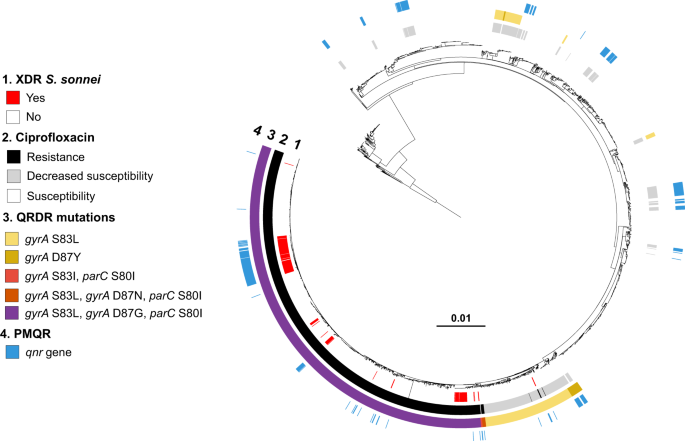
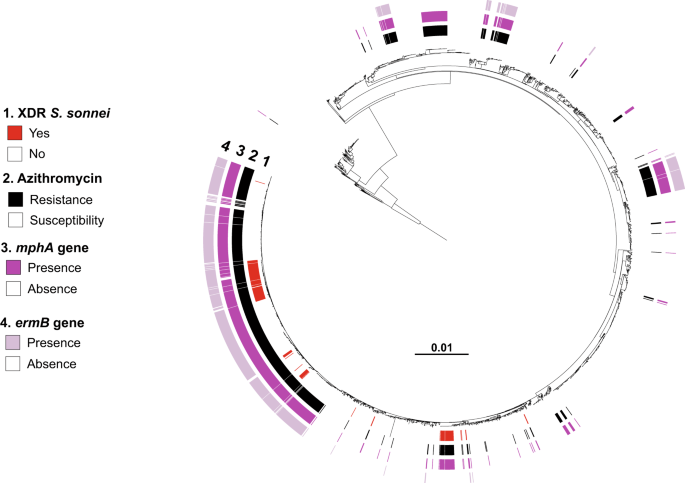
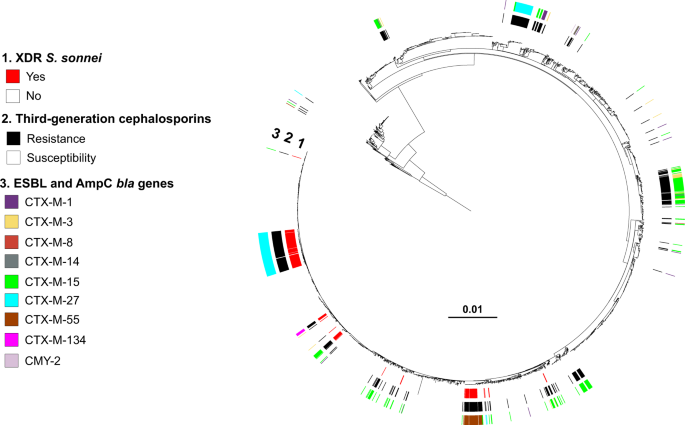
The combined phenotypic and genomic analyses revealed that all but four of the XDR S. sonnei isolates harbored three mutations in the quinolone resistance-determining regions (QRDR) of the gyrA and parC genes, conferring resistance to CIP (MIC ≥ 1 mg/L) (Figs. 2–4). The other four isolates—from the more ancestral genotypes, 3.6.1_CipR-parent and 3.6.1.1_CipR—harbored only one or two QRDR mutations, but also carried a plasmid-borne qnr gene, leading to CIP resistance. Resistance to AZM was conferred by the mphA and/or ermB genes, whereas resistance to 3GCs was conferred by various extended-spectrum beta-lactamase (ESBL) blaCTX-M genes (blaCTX-M-3, blaCTX-M-15, blaCTX-M-27, blaCTX-M-55, and blaCTX-M-134) (Figs. 2, 3, 5, and 6).
Maximum-likelihood phylogeny of 3141 S. sonnei genomic sequences as shown in Fig. 2. The rings show the associated information (see key) for each isolate, according to its position in the phylogeny, from the innermost to the outermost, in the following order: (1) the XDR isolates; (2) antimicrobial susceptibility testing for ciprofloxacin (resistance defined as minimum inhibitory concentration [MIC] ≥ 1 mg/L; susceptibility as MIC ≤ 0.06 mg/L; decreased susceptibility as MIC between 0.12 and 0.5 mg/L); (3) mutations in the quinolone resistance-determining region (QRDR) of gyrA and parC; (4) presence of plasmid-mediated quinolone resistance (PMQR) genes of the qnr family.
Maximum-likelihood phylogeny of 3141 S. sonnei genomic sequences as shown in Fig. 2. The rings show the associated information (see key) for each isolate, according to its position in the phylogeny, from the innermost to the outermost, in the following order: (1) the XDR isolates; (2) antimicrobial susceptibility testing for azithromycin (resistance defined as minimum inhibitory concentration [MIC] ≥ 32 mg/L; susceptibility as MIC ≤ 16 mg/L); (3) presence of the mph(A) gene; (4) presence of the erm(B) gene.
Maximum-likelihood phylogeny of 3141 S. sonnei genomic sequences as shown in Fig. 2. The rings show the associated information (see key) for each isolate, according to its position in the phylogeny, from the innermost to the outermost in the following order: (1) the XDR isolates; (2) antimicrobial susceptibility testing for third-generation cephalosporins (resistance defined as minimum inhibitory concentration [MIC] of ceftriaxone ≥ 4 mg/L; susceptibility as MIC ≤ 1 mg/L); (3) presence of bla genes encoding extended-spectrum beta-lactamases (ESBLs) or cephamycinases.
For better characterization of the mobile (or chromosomally integrated) elements encoding AMR, we also performed long-read sequencing on 16 XDR S. sonnei isolates (one to two isolates per XDR genomic cluster). The XDR S. sonnei isolates contained one to three plasmids carrying AMR genes (Supplementary Table 3). Plasmid sizes ranged from 8379 to 106,936 kb. A small ∼ 8 kb (either non-typable or typed as PTU-E63) plasmid encoding resistance to streptomycin (strA and strB genes), sulfonamides (sul2) and tetracyclines (tet(A)) was found in 12 of 16 XDR isolates from all four genotypes. This plasmid was highly similar to pMHMC-012 (GenBank accession no. CP053763) (Supplementary Fig. 1) from a S. sonnei isolate collected from a GBMSM patient in Boston, USA in 2017 (ref. 18).
Most of the other AMR plasmids belonged to IncFII (PTU-FE) and were related to pKSR100 (GenBank accession no. LN624486)—an epidemic MDR plasmid encoding resistance to AZM with a low fitness cost for its bacterial host — previously described in S. flexneri 3a, 2a, and S. sonnei sublineages associated with GBMSM networks from Europe, North America and Australasia10,15,19 (Fig. 3, Supplementary Table 3). A second, less common, plasmid encoding resistance to AZM was typed as IncB/O/K/Z; plasmids of this type were previously observed in XDR S. sonnei isolates in Southeast Asia6,7 (Fig. 3, Supplementary Table 3).
The ESBL blaCTX-M genes were located on multiple plasmids of different types (IncFII, including the pKSR100-like plasmid encoding resistance to AZM, IncB/O/K/Z, IncI1) or even integrated into the bacterial chromosome (Supplementary Table 3). Highly similar ESBL plasmids (98–99% nucleotide identity) were found in different XDR clusters (Supplementary Figs. 2–5). For example, an IncI1 (PTU-I1) plasmid carrying blaCTX-M-3 was identified in clusters X7 and X11 (same genotype, 3.6.1.1.2_CipR.MSM5) and an IncFII (PTU-FE) plasmid carrying blaCTX-M-27 was identified in clusters X2, X3 and X10 (two different genotypes, 3.6.1.1.1_CipR.SEA and 3.6.1.1.2_CipR.MSM5) (Fig. 3, Supplementary Table 2, and Supplementary Figs. 2 and 3). Some of our ESBL plasmids were also similar to certain plasmids described in previous studies. Hence, the blaCTX-M-27-carrying p202008564-6 plasmid (cluster X10, genotype 3.6.1.1.2_CipR.MSM5) displayed 99.6% nucleotide identity to p893916 (GenBank accession no. NZ_MW396858), from a S. sonnei isolate collected in London, UK, in 2020 (Supplementary Fig. 2)20. The second form of the ESBL plasmid found in cluster X10 isolates—and represented by p202008118-4—probably resulted from an insertion sequence (IS)-driven deletion or acquisition of the azithromycin-resistance gene, erm(B) (Supplementary Fig. 2). The blaCTX-M-134-carrying p202000562-4 plasmid (cluster X9, genotype 3.6.1.1.2_CipR.MSM5) displayed 90.3% nucleotide identity to p3123885 (GenBank accession no. CP049164), from a S. sonnei isolate acquired in Israel in 2019 (Supplementary Fig. 4)9. Finally, the blaCTX-M-3-carrying p201908234-4 plasmid (cluster X11, genotype 3.6.1.1.2_CipR.MSM5) displayed 99.7% nucleotide identity to p711-69 (GenBank accession no. CP049176), from a S. sonnei isolate acquired in Turkey in 2019 (Supplementary Fig. 3)9. In two S. sonnei isolates, 201701093 (cluster X1, genotype 3.6.1.1_CipR) and 201908033 (cluster X6, also 3.6.1.1_CipR), the blaCTX-M-15 ESBL gene was not located on a plasmid but on the bacterial chromosome (Fig. 3, Supplementary Table 3). In isolate 201908033, the ESBL gene was part of a ∼ 42 kb genomic island described in Supplementary Fig. 6, whereas in isolate 201701093, the ESBL gene was integrated into a ∼ 57 kb prophage sequence absent from other XDR genomes (Supplementary Fig. 7).
In the XDR genomic cluster X10, two isolates, 202110142 (accession no. ERR9940949) and 202111148 (ERR9941000) were phenotypically similar to other X10 isolates, except that they were susceptible to 3GCs (and were therefore not classified as XDR). However, they had the same antimicrobial drug resistance gene content (including blaCTX-M-27) as the other X10 XDR isolates. A careful inspection of the assemblies identified an IS (IS26) in both isolates, integrated at the same position of the blaCTX-M-27 promotor and probably leading to a non-functional ESBL gene. Together with the emergence of novel AMR genes, the presence of non-functional AMR genes with unaltered open reading frames (ORFs) constitutes a limitation of AMR prediction based exclusively on genomic data.
Our genomic analysis showed that this XDR phenotype emerged through a complex phenomenon of convergent evolution involving many AMR genes and structures. Studies of a larger number of XDR isolates by long-read sequencing might have revealed even greater diversity. Globally, the diversity of mobile and chromosomal AMR elements involved in XDR S. sonnei is undoubtedly greater. For example, blaCTX-M-14, an ESBL gene not encountered in our study, was identified in seven XDR S. sonnei isolates from Australia, UK, Vietnam and the US, between 2015 and 2019 (ref. 15).
One key element in the generation of the XDR phenotype was the acquisition of resistance to CIP, through fixed chromosomal mutations, in a successful sublineage (3.6.1) in South Asia at some time around 2007 (refs. 6,15). This sublineage subsequently and successively acquired resistance to AZM and 3GCs. These resistances were acquired independently, on multiple occasions (probably depending on the antibiotic selective pressure exerted in the different S. sonnei transmission networks and geographic areas), through the horizontal transfer of various plasmids and AMR genes. In the XDR S. sonnei isolates collected in France, IncFII pKSR100-like plasmids were frequently the vehicles of resistance to AZM, particularly in GBMSM-associated S. sonnei genotypes. Through their acquisition of an additional ESBL gene, these IncFII plasmids were also implicated in the resistance of most of our XDR isolates (clusters X2, X3, X5, X9, X10, and X12), as the vehicles of resistance to C3Gs, ultimately leading to the XDR phenotype. Interestingly, the virulence plasmid (pINV)—a ~210 kb low-copy number non-conjugative plasmid required for the invasion of host epithelial cells by S. sonnei—also belongs to the IncFII incompatibility group ([F27: A–: B–] replicon for pINV and [F35: A–: B–] replicon for pKSR100 according to pMLST 2.0 https://cge.food.dtu.dk/services/pMLST/)21,22,23. The coexistence of these two plasmids therefore suggests the existence of two types of selection pressure, mediated by type III secretion system (T3SS) expression24 and lipolysaccharide biosynthesis24 for the maintenance of pINV, and antibiotics for the maintenance of pKSR100.The great diversity of ESBL genes (blaCTX-M-3, blaCTX-M-15, blaCTX-M-27, blaCTX-M-55, and blaCTX-M-134) located on different plasmids (IncFII, IncI1, IncB/O/K/Z) or even integrated into the bacterial chromosome suggests that 3GC agents have recently exerted strong selective pressure on S. sonnei. For example, 3GCs are now recommended in place of AZM for the treatment of gonorrhea—a common sexually transmitted infection (STI) in GBMSM—in France and several other European countries25.
An emerging XDR S. sonnei strain
Our genomic analysis revealed that the 164 French XDR S. sonnei isolates were genetically diverse, but with one predominant cluster (X10) accounting for 62.2% (102/164) of XDR isolates. The genomic comparison of these 164 isolates collected in France with 67 previously published international XDR (or inferred to be XDR) S. sonnei isolates (Supplementary Table 4) revealed that 32.8% (22/67) of these international isolates were similar to the French XDR isolates from three clusters (X4, X9, and X10) (Supplementary Fig. 8). Eleven international isolates carrying the ESBL blaCTX-M-55 gene were identified in our X4 cluster. As expected for this cluster, which was associated with Southeast Asia, five isolates were collected in Vietnam in 2016 (01_0932, 01_0953, 01_0975, 01_1077, and 02_2142), three in Australia in 2016–2017 (AUSMDU00005736, AUSMDU00010002, and AUSMDU00006624; the two first being associated with travel to Southeast Asia), two were obtained in the UK in 2015–2016 (187596 and 261400, with no travel information); and one was obtained in the USA in 2017 (PNUSAE008493, with no travel information). Two international isolates carrying the ESBL blaCTX-M-134 gene were found to be similar to our X9 isolates. These two isolates were identified in 2019 in Switzerland (isolate 3123885, from a patient reporting travel to Israel) and in the UK (845732, with no travel information). Our largest XDR cluster, X10—belonging to genotype 3.6.1.1.2_CipR.MSM5 and containing the ESBL blaCTX-M-27 gene on a pKSR100-like plasmid—corresponds to the epidemic strain found in GBMSM in the UK and eight other European countries, with a total of 208 isolates obtained as of February 23, 2022 (ref. 5,26). At the time, France was the country with the largest number of isolates (n = 106) and the earliest isolate, dating from September 2020. All 73 XDR S. sonnei isolates obtained at the FNRC-ESS from January 1, 2022 to August 31, 2022 (a total of 336S. sonnei isolates were received over this time period) belong to genotype 3.6.1.1.2_CipR.MSM5 and contain the ESBL blaCTX-M-27 gene, suggesting that the epidemic X10 strain has continued its intensive circulation in France for a third year. This European epidemic strain was recently identified in the US and Australia25. A descriptive epidemiological study of the UK outbreak (72 cases) revealed that 21 of the 27 cases interviewed (78%) were HIV-negative GBMSM and users of HIV pre-exposure prophylaxis (PrEP)26. These individuals also reported having high-risk sex in England or Europe during the incubation period. Due to the few therapeutic options left, the recommended oral antimicrobial therapy, when required, was either pivmecillinam or fosfomycin (chloramphenicol not being readily accessible in the UK)26. For hospitalized or severe cases, carbapenems (colistin in case of allergy to beta-lactams) were used for three to five days, followed by an oral step-down treatment26. Further studies are required for a more detailed description of the clinical characteristics of these XDR S. sonnei infections (severity, existence of asymptomatic carriage, co-morbid conditions, including co-infections with other pathogens, such as monkeypox virus, etc.) and to confirm the efficacy of this antimicrobial treatment strategy. Post-exposure prophylaxis with doxycycline (Doxy-PEP) for the prevention of STIs in GBMSM is now the subject of heated debate27,28. A careful evaluation of the balance between the public health risks and benefits of this PEP will need to take the recent emergence of XDR S. sonnei into account, as high rates of resistance to tetracyclines (mediated by the two most common genes encoding tetracycline-specific efflux pumps, tet(A) and tet(B)) (Supplementary Tables 1–3) might provide these bacteria with a selective advantage, favoring their spread.
In conclusion, an effective national laboratory-based surveillance of Shigella infections, including antimicrobial susceptibility data, is therefore essential for informed decision-making and appropriate public health action to tackle the spread of these XDR S. sonnei strains currently circulating in different transmission networks. Genomic surveillance based on this new genotyping scheme with a common nomenclature will facilitate the detection and global tracking of these XDR S. sonnei strains of concern.