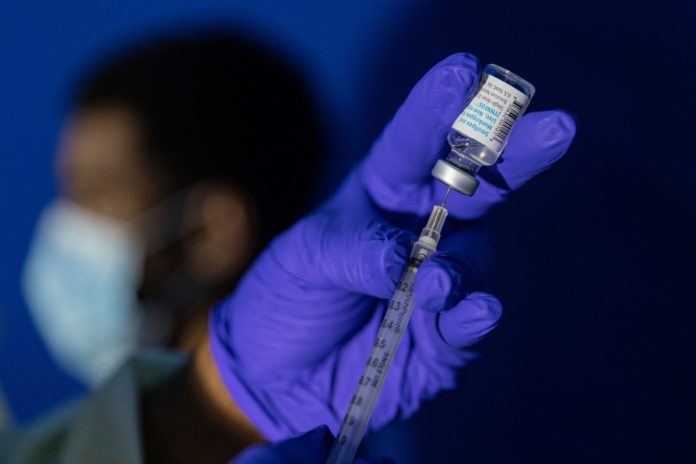
To address both the uncertain efficacy and scarce supply of the vaccine, federal health authorities have introduced a plan to maximize the number of people with some level of protection. Instead of being injected into fatty tissue, the Jynneos smallpox vaccine will now be injected intradermally — under the top layers of the skin — and in a much smaller amount, allowing one dose to be stretched into five doses. This technique, called “fractional dosing” or “dose sparing,” has been used in other vaccine campaigns, including influenza.
The Biden administration’s fractional dosing monkeypox vaccination plan is based on results from a single study and remains subject to scientific and clinical debate. It may produce useful data about how intradermal vaccination produces an immune response. However, it also adds to the experimental nature of the vaccine rollout.
Past health crises help illustrate how medical scarcity and government neglect can induce people to participate in medical and public health projects with uncertain outcomes. Monkeypox vaccination is the latest chapter of this history of experiments in health — a history that ties biomedical uncertainty to social marginalization.
The growing influence of bioethics in the mid-20th century, particularly after the horrors of Nazi medicine and the Tuskegee syphilis study, normalized informed consent in research. Consenting to participate in medical research sometimes brought access to treatments. Yet such research didn’t always address unequal health-care access.
This was the case in tuberculosis treatment experiments among Navajo people in the 1950s. White researchers learned of an outbreak of tuberculosis at the Many Farms community within the Navajo Nation in Northeastern Arizona (Dá’ák’eh Halani). The researchers hoped to test the efficacy of treating TB with the antibiotic isoniazid. But to understand isoniazid’s unique efficacy, they needed to test it on a population that did not have access to additional antibiotics for TB. If other antibiotics were in use, it would be difficult to show isoniazid’s individual effects. Due to years of U.S. government neglect, the people living at Many Farms fit this criteria, and therefore seemed ideal for studying isoniazid. The community agreed to the research.
The drug helped address the TB outbreak, but the focus on the efficiency of isoniazid meant the intervention did not address underlying health-care inequalities. Without investments in the broader health of the community, mortality remained high, and the medical researchers undermined community trust in health-care workers. The experiment was extractive. It built knowledge but denied care.
By the 1960s and 1970s, conversations about addressing root causes of disease and disparities in health-care access through research design were increasingly common. Activists became more involved in debates about who could participate in novel medical and public health research, particularly in areas of women’s health and cancer.
For example, women’s health groups such as the Boston Women’s Health Collective sought to change the long-held reluctance of clinical trials to enroll women.
Drug trials for HIV/AIDS in the 1980s marked another major turning point when activists called out the links between research and health-care access. The impact of AIDS on gay men in the 1980s in the United States meant that gay activists built movements that demanded access to experimental antiretroviral drugs. When scientists conducted a clinical trial of AZT (azidothymidine) and concluded that it could reduce HIV transmission and death in people with AIDS, the drug quickly became a rallying point for some AIDS activists. Being part of testing the drug’s efficacy became a desperately desired chance for many HIV-positive people to live on.
But some activists and physicians questioned disparities among participants in the AZT trial. They noted that trial participants who had higher rates of survival and fewer adverse reactions were also more likely to have health insurance and access to their own physicians. Regular medical support and monitoring was critically important for preventing death. At trial sites where participants didn’t have consistent health-care access, more deaths occurred. The AZT trials illustrated the stakes of overlooking potentially meaningful differences in participants’ access to health care.
Clinical trials for HIV drugs also often under-enrolled Black and Latino participants, and overrepresented White gay men.
Activists pointed out that efficacy actually had two pieces — the effectiveness of the drug and access to care.
Coalitions of AIDS activists in the 1990s made the latter issue the foundation for their demands of public health officials and medical professionals. This was especially the case for groups of African American activists focused on improving HIV care in Black communities.
By connecting to global campaigns on issues such as drug pricing, patents and the availability of cheaper generic medicines, these groups connected biomedical innovation to the reduction of health-care access inequalities. Activists also challenged the pharmaceutical industry, questioning how its profit motives shaped experimental priorities. Bodies could evidence far more than biological truths; they were staging grounds for social and economic dilemmas.
The efficacy of preventive measures took a different turn in the 2010s, during debates about the development of pre-exposure prophylaxis, or PrEP, which helps prevent HIV transmission. The drug known as Truvada, taken once daily and now available as a generic, can reduce the possibility of HIV transmission up to 99 percent. It was approved by the FDA in 2004 as a treatment for people living with HIV and in 2012 as pre-exposure prophylaxis in people who are HIV-negative.
Despite the drug’s efficacy, some early public reactions to the drug nonetheless described it in terms of uncertainty. Instead of asking whether the drug worked, discussions often centered on how people would behave if they had access to the drug. While PrEP’s efficacy was clear, some health authorities expressed concerns that the drug would lead to increased sex without condoms, and the risk of higher STI prevalence.
Arguments about morality obstructed the rollout of the drug, leaving some of those most at risk for acquiring HIV less able to access the drug. Meanwhile, pharmaceutical marketing tended to target White gay men. Indeed, this has resulted in uneven access to PrEP among Black men and women.
These histories show that beyond the critical question of “does this medicine work?” an additional question is crucial: how to ensure care for people participating in uncertain biomedical projects, especially when due to government failure?
Monkeypox illustrates the stakes of both these questions. Activists argue that the new fractional dosing plan may expand the sheer number of doses, but does not necessarily ensure access for Black and Latino men at a time when test positivity is increasingly concentrated in communities of color.
There are also global inequities. To date, not a single Jynneos vaccine has been made available in West and Central Africa, where monkeypox has resulted in far more deaths than in those countries with vaccine access.
Still, in places where vaccines have been distributed, there is reason to be cautiously optimistic about the sprint to contain and treat monkeypox. Data published recently from Great Britain, Canada, Germany and New York suggest that the incidence of new monkeypox cases may be decreasing. It is likely that alongside vaccines, both shifting sex practices and the work of community-led research by health advocates are contributing to this decline.
The past teaches us that efficacy is a problem that is always both scientific and social. As experiments in health continue, it is crucial to remember whose bodies make certainty possible — and at what costs.