The UK Health Security Agency (UKHSA) is working with the NHS and the public health agencies of the 4 nations to investigate the monkeypox outbreak in the past few weeks. This briefing is produced to share data useful to other public health investigators and academic partners undertaking related work. It includes early evidence and preliminary analyses which may be subject to change.
Potential levels of the outbreak in England
The outbreak can be considered to fall into one of 4 potential levels of transmission. These may be refined with better understanding of modes of transmission. At present, England is judged to be in Level 2 and we are monitoring closely for any evidence of Level 3.
Level 1
Incursions from rest of the world – small numbers of imported cases with limited onward transmission.
Level 2
Transmission within a defined sub-population with high number of close contacts.
Level 3
Transmission within multiple sub-populations or larger sub-population.
Level 4
Wider significant community transmission – with potential for endemic and local epi-zoonotic disease.
Summary
Up to 8 June 2022 there were 336 laboratory confirmed cases of monkeypox in the UK. A high proportion of cases are London residents, and the majority are male.
Given the reporting delay from symptom onset to case ascertainment, we cannot yet determine if transmission has stopped increasing.
Detailed case interviews show that traditional contact tracing is currently challenging, but these interviews identify transmission networks, risk factors and behaviours that can be used to tailor public health communications and target intervention delivery. Epidemiological analysis will identify if these patterns change.
Preliminary estimate of the serial interval is 9.8 days though with high uncertainty (95% credible interval, 5.9 to 21.4).
UKHSA has commenced sharing of genomic data. However, it is recommended that monkeypox genomic data conventions are agreed, including sharing of raw as well as consensus data, given the potential for variation in bioinformatics pipelines and the impact that this may have on downstream analysis.
The mutations specific to the current global outbreak clade are distributed across the genome. There is a small subset of mutations in proteins that are involved in virus transmission, virulence, or interaction with antiviral drugs. Structural modelling is required to assess the impact of the individual mutations on the protein in the first instance.
UKHSA has produced a summary of evidence gaps and is working with partners to address these. UKHSA has convened an expert group of public health, NHS and academic partners to steer a comprehensive investigation.
Part 1. Research and evidence gaps prioritisation
UKHSA has carried out a research and evidence gaps analysis relating to the monkeypox outbreak in the UK. We are working collaboratively with academic partners, including National Institute for Health Research Health Protection Research Units and national research funders, to develop and implement rapid studies to address these. The priority evidence gaps are shown in Table 1.
Table 1. Priority research and evidence
| Research topic | Priority evidence gaps |
|---|---|
| Surveillance | Levels of undiagnosed disease Trends and growth Level of asymptomatic infection Wastewater surveillance |
| Transmission dynamics | Transmission risk to contacts Modes of transmission |
| Biological characterisation and virology | Genome sequencing and in-host variation Viral dynamics Virus characterisation, including biological significance of mutations |
| Clinical characterisation | Clinical presentation and outcomes. Groups at risk of worse outcomes |
| Vaccine response and immunology | Immune response to infection and vaccines Immunological correlates of protection Post-implementation effectiveness |
| Therapeutics | Post-exposure Prophylaxis with Tecovirimat Early treatment and risk of transmission Impact on disease protection |
| Diagnostics and evaluation | Best site to test Home sampling and testing Evaluation of Lateral Flow Devices Development of serology test |
| Evaluation of other interventions | Effectiveness of contact tracing |
| Behavioural and other social sciences | Public perception of risk Public understanding of disease Help seeking behaviour Vaccine acceptability Adherence to self-isolation Media coverage, behaviour and stigmatisation |
| Longer term consequences of infection | Are there longer-term consequences of infection? |
| Other | Reverse zoonosis risk |
Part 2. Epidemiology update
2.1 Current epidemiological situation
Cases of monkeypox infection were confirmed in England from 6 May 2022. The outbreak has mainly been affecting people without documented history of travel to endemic countries. Up to 8 June 2022 there were 336 laboratory confirmed cases in the UK. Of the confirmed cases 11 were in Scotland, 2 in Northern Ireland, 3 in Wales and 320 were in England.
Table 2. Number of laboratory confirmed cases by devolved administrations, 6 May to 8 June 2022 (n=336)
| Devolved administrations | Confirmed cases |
|---|---|
| England | 320 |
| Northern Ireland | 2 |
| Scotland | 11 |
| Wales | 3 |
| Total | 336 |
A high proportion of England cases were known to be London residents (81%, 224 of 276 with reported home address), see Table 3. Where gender information was available, 311 (99% of 314) confirmed cases were male, with 3 confirmed female cases. The median age of confirmed cases in the UK was 38 years old (interquartile range 32 to 44).
One hundred and fifty-two cases participated in more detailed questionnaires, implemented from 26 May 2022, and used retrospectively. In this data, 151 of the 152 men interviewed identified as gay, bisexual and other men who have sex with men (GBMSM), or reported same sex contact, and the remaining individual declined to disclose this information. Recent foreign travel, within 21 days prior to symptom onset, was reported by 75 cases (22%), with 59 of these reporting travel within Europe.
Figure 1 demonstrates symptom onset dates of confirmed cases in England, where known. The median reporting delay, calculated here as the time between symptom onset date and date notified to UKHSA, was 10 days for cases newly reported in the week starting 6 June 2022. The reporting delay has improved since early in the incident, from 12 days for cases reported in the week starting 16 May 2022.
Table 3: Number of laboratory confirmed monkeypox cases by region of residence, England, March to 8 June 2022 (n=320)
| Region of residence | Total confirmed |
|---|---|
| East of England | 10 |
| East Midlands | 3 |
| London | 224 |
| North East | 2 |
| North West | 11 |
| South East | 15 |
| South West | 2 |
| West Midlands | 6 |
| Yorkshire and Humber | 3 |
| Unknown* | 44 |
| Total | 320 |
*Address not yet confirmed.
Figure 1. Incidence of confirmed monkeypox cases by day of symptom onset in England as of 8 June 2022
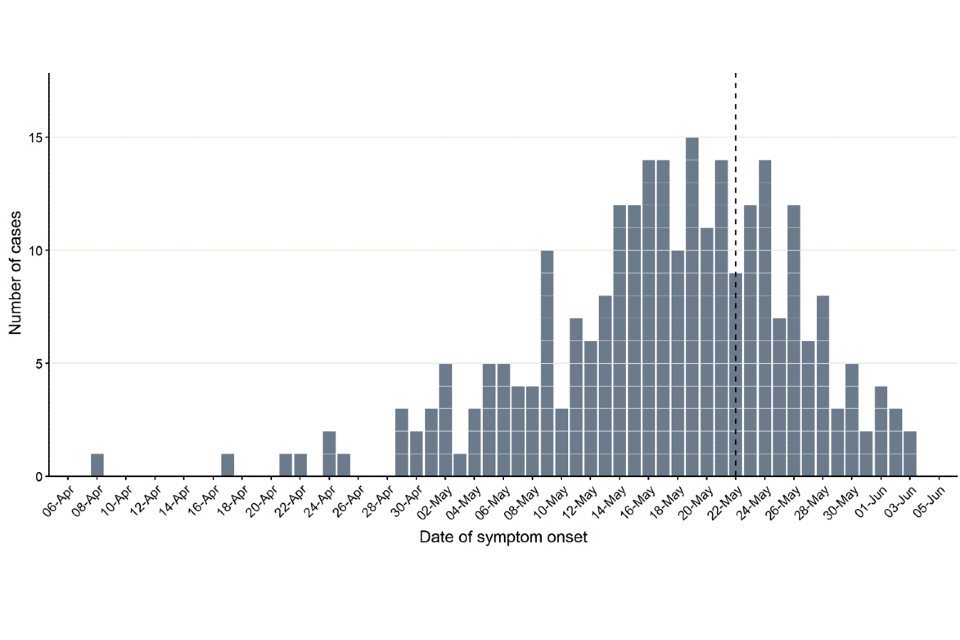
N = 265 (onset dates missing for 55 of 320 cases); reporting patterns suggest that data to the left of the dotted line are largely complete despite reporting delays.
The data used in this graph can be found in the accompanying spreadsheet.
2.2 Findings from rapid sexual health questionnaires
To understand the epidemiology of the current pattern of monkeypox transmission in the UK, UKHSA retrospectively re-interviewed confirmed cases. Cases were invited for re-interviews focused on their sexual health, which were conducted via phone calls between 25 and 30 May 2022 using structured questionnaires. Of the 82 cases with known or suspected links to transmission in GBMSM sexual networks identified up to 25 May 2022, 45 (55%) participated in re-interviews using this specific detailed questionnaire. Reasons for not participating in these re-interviews included lack of contact details (n=11), declining to participate (n=4) and inability to establish phone contact for re-interview (n=22). All interviewed cases were cisgender men and identified as gay (n=40), bisexual (n=4), or in another way (n=1).
Symptom onset dates ranged from 8 April to 20 May 2022 with all cases diagnosed in May 2022. Analysis of onset, travel, and venue attendance dates found that 31 (69% of 45) interviewed cases with no travel abroad had symptom onset dates from April 2022 onwards, including one case who subsequently travelled to an international event. Throughout May 2022, several cases returning to the UK after travelling in Europe developed symptoms.
Findings show that monkeypox is being transmitted in geographically diffuse sexual networks. Nearly all (98%) of interviewed cases reported sex with other men during the incubation period (5 to 21 days). The majority (60%, 27 out of 45) were diagnosed with an STI in the previous year, 44% (20 out of 45) reported more than 10 sexual partners in the previous 3 months, and 44% (20 out of 45) reported group sex during the incubation period. Sexual networks involved in this outbreak are interconnected both within and outside the UK, as 30% (13 out of 45) of cases reported sexual activity in cities other than their place of residence, 20% (9 out of 45) reported sexual activity abroad, and 24% (11 out of 45) reported sexual activity with men who are not UK residents during the incubation period.
Findings from these interviews highlighted specific challenges and potential opportunities for outbreak control.
Traditional contact tracing as a primary control intervention in this specific group will be challenging as most cases reported having sexual contact with new or casual partners, sometimes in the context of cruising grounds or during chemsex, frequently where contact details were unavailable for tracing.
Sexual health services (SHS) were identified as potential settings for delivering opportunistic control interventions, such as vaccines, since there are established links between people at risk of infection and these services. Most cases were human immunodeficiency virus (HIV) negative and 91% (29 out of 32) of them were linked to SHS for the delivery of HIV pre-exposure prophylaxis (PrEP). All cases who were living with HIV were linked to HIV care and were on treatment; with more than 90% reporting undetectable viral loads.
Of those interviewed, 44% (20 out of 45) reported attending sex-on-premises venues in the UK or abroad during the incubation period, such as saunas, dark rooms, or sex clubs. Therefore, collaborating with sex-on-premises venues to implement targeted interventions would support outbreak control. A minority of cases reported sexual activity in international festivals or events. However, 36% (16 out of 45) did not report attending any sex-on-premises venues, festivals or events in the 3 weeks before symptom onset, suggesting the need for wider interventions that reach all GBMSM in these sexual networks.
There was a high usage of geospatial dating applications, with 64% (28 out of 45) of cases reported meeting new partners during the incubation period using such apps, indicating these are likely useful platforms for disseminating health promotion messages or supporting innovative approaches to contact tracing.
Findings from this early rapid sexual health questionnaires have some limitations. It is possible that cases who participated in interviews have different sexual behaviour and risks than those who declined participation. When comparing interviewed and non-interviewed cases, there were no significant differences in age, region of residence, or reported travel history. Nonetheless, these findings might not be representative of the totality of the outbreak due to participation bias and evolving patterns of transmission, affecting their generalisability. Analyses from ongoing case interviews will indicate whether these patterns are changing; for example, the proportion of cases who do not report sexual activity or attendance at festivals and venues.
Part 3. Genomics
3.1 Sequencing and bioinformatics
Monkeypox encodes an approximately 197kb base pair double-stranded deoxyribonucleic acid (DNA) genome with an average guanine-cytosine content (GC) of 33%. It contains 190 coding sequences. Monkeypox genomes have a conserved central region containing housekeeping genes, with the accessory genome located at the ends, with a typical inverted terminal repeat at each end.
The monkeypox genome structure poses some challenges for accurate reconstruction; the genome contains a long (approximately 6.5kb) inverted tandem repeat which is hard to resolve into individual copies with short read approaches. The low GC of the genome results in long poly-A/T homopolymeric tracts which can be hard to accurately sequence. Additionally, local tandem repeats are scattered across the genome which can pose assembly challenges.
The slow evolutionary rate and recent common ancestor of the current outbreak makes genomic epidemiology over short time scales contingent on reliable sequence reconstruction for accurate inferences: this may be higher than the achievable accuracy of sequencing platforms due to read length or consensus accuracy and will require characterisation and regular validation. An additional concern for genomic epidemiology is the potential for within-host diversity both within and between lesions. Defining this diversity is essential to inform the use of genomics in the analysis of the outbreak.
Accurate genome reconstruction depends on achieving sufficient depth and breadth of coverage. So far, genome sequences have been generated primarily through metagenomic approaches which sequence the contents of a clinical sample without targeting. Feasibility of metagenomics approaches for generating useful sequences are typically limited by the ratio of host to virus reads in the sample which can be partially mitigated through very deep sequencing approaches.
UK experience suggest that good genome coverage is achievable through this method up to a cycle threshold (Ct) value of around 20, with partial genomes being seen up to Ct of 25. This means that it will not be possible to sequence all cases using this approach. In future, targeted methods such as bait capture or amplicon methods may be able to increase sequencing sensitivity and so increase the yield from sequencing. However, given metagenomics methods are untargeted they may be favourable whilst outbreak diversity remains uncertain. Other physical enrichment measures may increase metagenomics sensitivity. Enrichment can also be achieved through culture isolation, but this may generate spurious mutations and is not recommended as the primary approach.
Bearing in mind these issues we recommend that:
- both sequence read data and consensus sequences should be shared to permit consistent bioinformatics approaches
- a conservative approach to variant calling avoiding repetitive and low-complexity regions should be employed
- bioinformatics approaches should be tuned for specific data type and cross-validated between sample preparation, sequencing platforms and assembly methods
- combined epidemiological and genomic analysis will be needed to interpret fine-scaled phylogenies with confidence.
UK genomic data sharing has commenced to Genbank.
3.2 Biological implications of the genome findings
The current UK monkeypox strain contains 48 single mutations in its genome relative to the 2018 UK monkeypox strain. Twenty-seven of these mutations are silent in that they do not change any of the viral proteins. Twenty-one of the mutations cause changes in viral proteins.
Mutations are classed in our assessment as low, medium and high priority for investigation, based on the known function of the viral proteins. These assessments are based on the effect of full deletion of these proteins from other orthopoxviruses. It is important to qualify that at present we cannot predict the effect of single mutations compared to full protein deletions. There are 2 mutations of low priority:
- C9L(R48C): antagonist of some interferon stimulated gene products, when deleted from vaccinia virus, the virus replicates worse in the presence of interferon
- A46L(H221Y): virulence factor, deletion of the gene from vaccinia virus reduces virus virulence in mice
There are 4 mutations at medium priority:
- C23L(S105L): Chemokine binding protein, deletion of this gene from rabbitpox virus increases disease severity in rabbits
- C22L(S54F): Tumour necrosis factor (TNF) receptor-like protein – deletion of a similar gene from ectromelia virus increases lung pathology in mice
- C19L(D266N): Ankyrin repeat protein (unknown function-might be host range or virulence)
- F13L(E353K): Target of antiviral drug tecovirimat – Tecovirimat resistance has been shown to be conferred by a single mutation at F13L(G277C)
There are 3 mutations in one protein which are classed as high priority:
- B21/B22(D209N, P722S, M1741I): T-cell inhibitor also found in cowpox virus, camelpox virus and horsepox virus, knock-in of this protein into non-virulent cowpox strains increased disease severity and mortality in rats.
Whilst outbreak clade mutations are distributed across the genome, there is a small subset of mutations in proteins that are involved in virus transmission, virulence or interaction with antiviral drugs. Structural modelling is required to assess the impact of the individual mutations on the proteins.
Part 4. Transmission dynamics
At this early stage of the outbreak the ability to infer underlying transmission dynamics is limited. Estimating the current number of active cases depends heavily on assumptions about ascertainment of non-GBMSM cases.
The reproduction number, R, is highly uncertain especially because of the stochastic nature of transmission and plausible values for overdispersion. In addition, it is possible the outbreak might have impacted the behaviour of the affected populations. Although the apparent plateau in incidence shown in Figure 1 might indicate that transmission is slowing, the backfilling of cases (that is, reporting cases today that first experienced symptoms several weeks ago from delayed ascertainment of initial cases) means that the current incidence trajectory is uncertain. The median delay between symptom onset and cases appearing in the UKHSA data is 10 days. However, the maximum delay has been recorded at over 5 weeks, related to case finding and re-testing of residual samples. The trajectory of the epicurve (in Figure 1) should become clearer in future weeks.
In preparation for further modelling work, UKHSA has started to estimate various parameters of the disease.
4.1 Serial interval
The current serial interval for monkeypox in the UK was estimated using a doubly interval censored model adjusting for right truncation (Ward and Johnsen, 2021, Overton and Ward, 2021).
Seventeen matched exposer-exposee pairs with known symptom onset dates were analysed. Without correcting for right-truncation in the data, where longer serial intervals are missing at the end of the time-series, the mean serial interval time is 6.4 days (95% credible interval (CI) 4.8 to 8.9), see Figure 2a. After correcting for right-truncation, the mean serial interval time was estimated at 9.8 days (95% CI 5.9 to 21.4), as shown in Figure 2b.
Due to the small sample size, there is currently high uncertainty in the mean serial interval, particularly when attempting to correct for the right-truncation (see Figure 3).
Figure 2. Estimates of the mean and standard deviation of the serial interval of monkeypox in the UK
Doubly interval censored modelling a) of the serial interval mean and standard deviation with 95% CI and b) adjusted for right truncation of the serial interval mean and standard deviation with 95% CI.
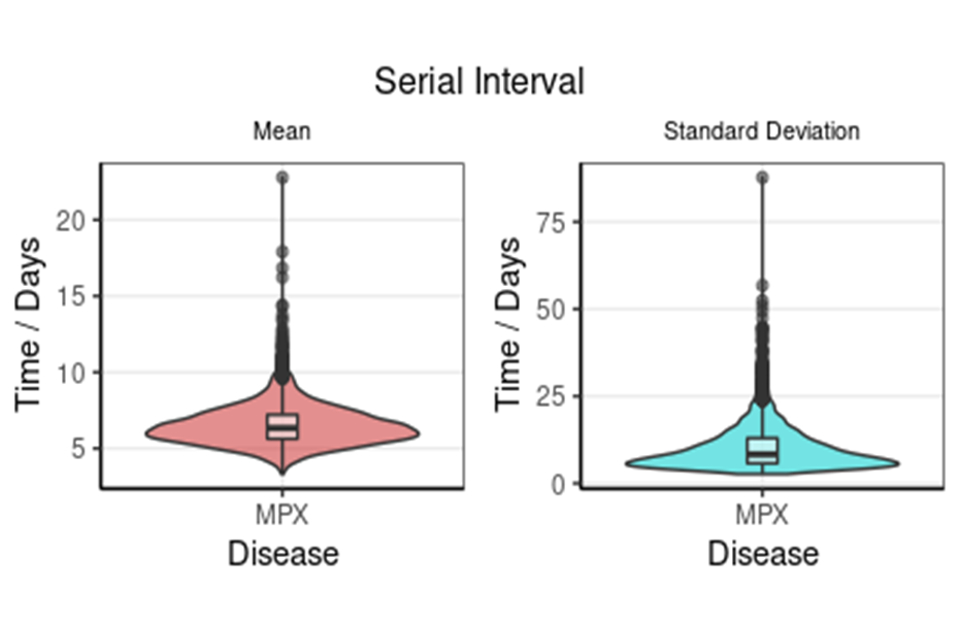
Figure 2a
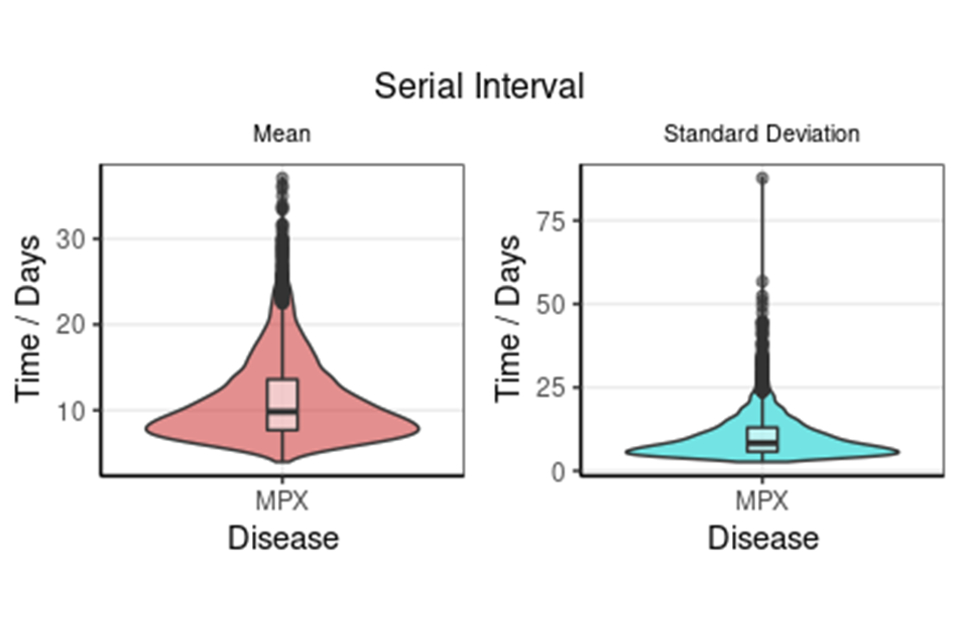
Figure 2b
Supplementary data is not available for these figures.
Figure 3. The monkeypox serial interval distribution from the doubly interval censored model adjusted for right truncation
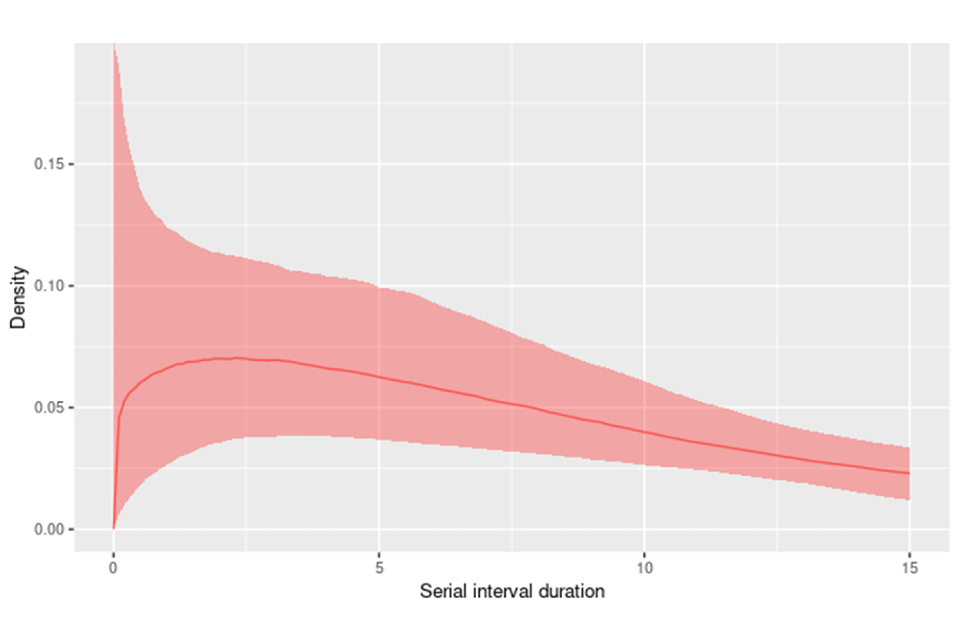
Supplementary data is not available for this figure.
Sources and acknowledgments
Data sources
Monkeypox virus PCR results are submitted to UKHSA daily by the Rare and Imported Pathogens Laboratory, Porton. Data on people testing positive since 6 May 2022 is enhanced with demographic, symptom, and exposure information extracted from the UKHSA Health Protection Team case management system (HPZone), or collected during detailed case interviews.
Authors of this report
Charlotte Anderson, Carolina Arevalo, Sooria Balasegaram, Jessica Bridgen, Chloe Byers, Meera Chand, Hannah Charles, Rachel Christie, Fergus Cumming, Paula Blomquist, Katie Wrenn, Irene Gonsalvez, Susan Hopkins, Nicholas Loman, Jason Mercer, Isabel Oliver, Christopher Overton, Mateo Prochazka, Steven Riley, Cian Ryan, Henry Simkins, Amoolya Vusirikala, Thomas Ward, William Welfare.
Contributors
UKHSA Data, Epidemiology and Analytics Cell
UKHSA Research and Science Cell
UKHSA Modelling Cell
UKHSA Genomics Public Health Analysis
UKHSA Sexual Health Liaison Group
UKHSA Monkeypox Incident Management Team
Acknowledgements
The authors are grateful to those teams and groups providing data for these analyses including:
- British HIV Association
- British Association for Sexual Health and HIV
- International Severe Acute Respiratory and emerging Infections Consortium (ISARIC)
- Sexual Health Services
- NHS England and Improvement
- High Consequence Infectious Diseases Network
- Public Health Scotland
- Public Health Wales
- Public Health Agency, Northern Ireland
- University of Birmingham