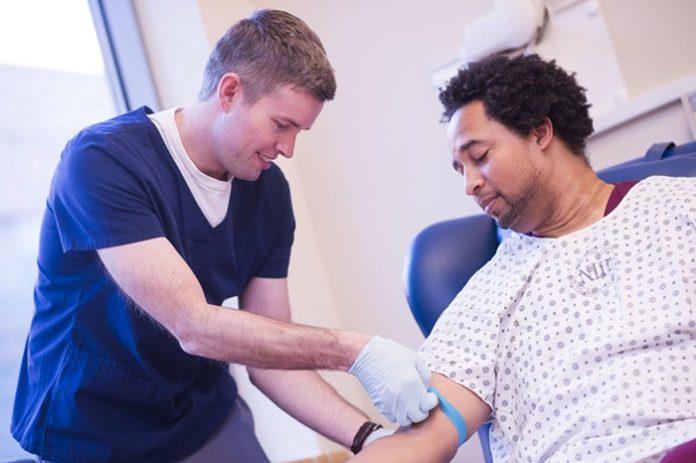
You’re eligible for the study if you’re a gay or bisexual man in South Florida, who is between 18 and 39 years old and has had sex with another man within the past three months.
After four decades, the U.S. Food and Drug Administration has signaled it is open to possibly doing away with the homophobic policy it has used to deny millions of gay and bisexual men the chance to donate lifesaving blood and plasma.
OneBlood, a major Florida-based blood center, is recruiting men across the Greater Miami area and as far north as Palm Beach County to participate in an FDA-funded, first-of-its-kind study aiming to identify alternatives to the current intake questionnaire, which asks prospective male donors whether they have had sexual contact with another man. Men who respond affirmatively are turned away if the last time they had sex with a man was within the past three months.
The study, known as ADVANCE (Assessing Donor Variability And New Concepts in Eligibility), is recruiting 2,500 participants from seven other major cities across the country: Atlanta, Los Angeles, Memphis, New Orleans, Orlando, San Francisco, and Washington D.C. Also helping spearhead the study are two other major blood organizations: the American Red Cross and Vitalant.
Study participants can earn up to $85. The process includes showing up at OneBlood’s Cypress Creek location, filling out a questionnaire, and having two blood samples drawn, says Susan Forbes, a spokeswoman for OneBlood. You’re eligible for the study if you’re a gay or bisexual man in South Florida, who is between 18 and 39 years old and has had sex with another man within the past three months.
“This is a big deal,” Forbes says. “It’s a study that we’ve been waiting for It’s a policy that has been out there for many years, and this is a moment where [participants] are having an opportunity to participate in making change, and it’s significant.”
The overarching goal, Forbes tells New Times, is to push the FDA to modify its screening questionnaire — required to be completed by all donors regardless of sexual orientation — to include questions that determine individual risk as opposed to closed-ended questions that automatically disqualify a person if they answer a certain way.
One of the earliest Florida participants in the study is renowned influencer Blake Lynch, 30, who is openly gay and a registered nurse living in Orlando with his husband of five years. His followers know him better as “Nurse Blake” on social media, where he regularly (and hilariously) sounds off on his experiences as a healthcare professional.
In an interview with New Times, Lynch says he felt compelled to participate in the study after being turned away from a blood donation site in 2013 after indicating he was gay. After the experience, Lynch formed a social campaign called Banned4Life, which sought to bring awareness to the fact that all gay and bisexual men at the time were barred from donating.
“This is the closure of a circle I’ve been waiting for — for us to be able to present evidence to the FDA that our blood can save lives, that we should not be banned, and we need to make a change based on science,” Lynch says. “I think this study is going to be able to show hard proof on why there should be a change in the policy and how there can be a change to the policy while still ensuring the safety of blood products.”
In 1983, the FDA implemented a lifetime donation ban for gay and bisexual men, collectively referred to as “MSM,” or “men who have sex with men.” The controversial and widely denounced policy is one that is rooted in the early days of the AIDS crisis and has existed in one form or another ever since.
In 2015, the federal government revised its policy to state that men who have sex with men were eligible to donate — if they were celibate for one year.
As the coronavirus pandemic began to overtake the United States last year and donation inventory reached dangerous lows, the federal government scaled back the deferral period from one year to three months in a desperate effort to broaden the donor pool. Between March and April 2020, the American Red Cross was forced to axe 13,000 blood drives because of stay-at-home orders, the organization confirmed to New Times last year.
“OneBlood, like all blood centers, has to follow the rules and regulations the FDA puts in place. So we don’t make the rules, but we have to follow them,” Forbes says. “We are always supportive of more people being able to enter into the blood pool. Any time more people can be eligible to donate blood that’s a good thing.”
Those interested in learning more or signing up for the paid study can do so here.