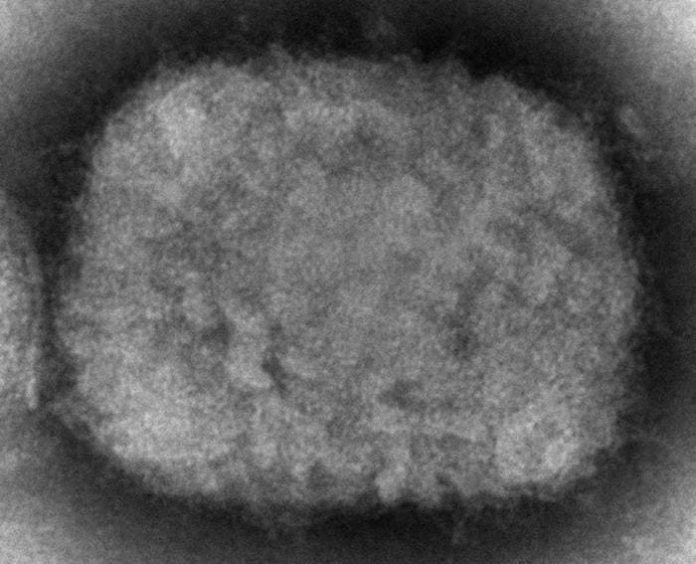
LONDON – The European Medicines Agency says it will begin reviewing data to decide if a smallpox vaccine made by the pharmaceutical company Bavarian Nordic might also be authorized for monkeypox, amid a growing outbreak of the disease across the continent.
In a statement on Tuesday, the EU drug regulator noted that the vaccine, known as Imvanex in Europe but sold as Jynneos in the U.S., is already cleared for use against monkeypox by American regulators.
In Europe, the vaccine is only authorized in adults for the prevention of smallpox, which is related to monkeypox.
“The decision to start this review is based on results from laboratory studies suggesting that the vaccine triggers the production of antibodies that target the monkeypox virus,” the EMA said. The regulator said that supplies of the vaccine are currently very limited in Europe.
According to the U.S. Centers for Disease Control and Prevention, there are currently more than 4,300 cases of monkeypox globally, of which the majority are in Europe.
The vast majority of cases are in gay and bisexual men, and in other men who have sex with men, although scientists warn anyone who is in close, physical context with someone who has monkeypox or their clothing or bedsheets is at risk of infection.
Several countries including Britain, Germany and the U.S. have already begun vaccinating those at highest risk of catching monkeypox using smallpox vaccines. Although the disease has been endemic in parts of Africa for decades, vaccines have not been used to stamp out previous outbreaks there.
Britain, which has the biggest outbreak beyond Africa, recently widened its vaccination policy to those at highest risk of monkeypox, which it defined as “gay and bisexual men at higher risk of exposure,” meaning those who have “multiple partners, participate in group sex or attends ‘sex on premises’ venues.”
On Tuesday, Britain’s Health Security Agency said there were now 1,076 cases of monkeypox across the country, adding that they expected to see infections rise further “in the coming days and weeks.”
Last week, the agency said the outbreak was spreading only in “defined sexual networks of gay, bisexual, or men who have sex with men.”
Most monkeypox patients experience only fever, body aches, chills and fatigue. People with more serious illness may develop a rash and lesions on the face and hands that can spread to other parts of the body. Most people infected with monkeypox recover within weeks without needing medical care but the disease can be more severe in vulnerable populations, like pregnant women and children.
The World Health Organization declined last week to declare monkeypox a global emergency, but has said it is working on a vaccine-sharing mechanism that some fear could see vaccines go to rich countries like the U.K. that already have their own stockpiles.
According to the health analytics firm Airfinity, Britain has 4.500 doses of the Bavarian Nordic vaccine and has ordered another 20,000 shots. The U.S. has received more than 1 million doses already and has ordered another 13 million.
On Tuesday, EU health commissioner Stella Kyriakides said the first deliveries of vaccines were arriving to the continent’s “most affected countries,” with more than 5,000 doses arriving in Spain, out of more than 109,000 procured.
The EU said in a statement that member countries with the most cases were being prioritized for deliveries; Portugal, Germany and Belgium will be the next countries to receive doses.
To date, there have been more than 1,500 monkeypox cases in Africa, with at least 66 deaths. No deaths beyond Africa have been detected.
___
Samuel Petrequin in Brussels contributed to this report.