Summary
Background
The early epidemiology of the 2022 monkeypox epidemic in non-endemic countries differs substantially from the epidemiology previously reported from endemic countries. We aimed to describe the epidemiological and clinical characteristics among individuals with confirmed cases of monkeypox infection.
Methods
We descriptively analysed data for patients with confirmed monkeypox who were included in the GeoSentinel global clinical-care-based surveillance system between May 1 and July 1 2022, across 71 clinical sites in 29 countries. Data collected included demographics, travel history including mass gathering attendance, smallpox vaccination history, social history, sexual history, monkeypox exposure history, medical history, clinical presentation, physical examination, testing results, treatment, and outcomes. We did descriptive analyses of epidemiology and subanalyses of patients with and without HIV, patients with CD4 counts of less than 500 cells per mm3 or 500 cells per mm3 and higher, patients with one sexual partner or ten or more sexual partners, and patients with or without a previous smallpox vaccination.
Findings
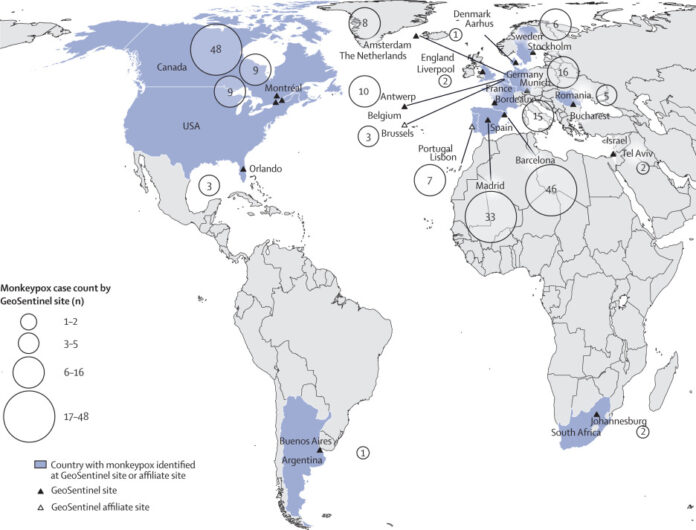
226 cases were reported at 18 sites in 15 countries. Of 211 men for whom data were available, 208 (99%) were gay, bisexual, or men who have sex with men (MSM) with a median age of 37 years (range 18–68; IQR 32–43). Of 209 patients for whom HIV status was known, 92 (44%) men had HIV infection with a median CD4 count of 713 cells per mm3 (range 36–1659; IQR 500–885). Of 219 patients for whom data were available, 216 (99%) reported sexual or close intimate contact in the 21 days before symptom onset; MSM reported a median of three partners (IQR 1–8). Of 195 patients for whom data were available, 78 (40%) reported close contact with someone who had confirmed monkeypox. Overall, 30 (13%) of 226 patients were admitted to hospital; 16 (53%) of whom had severe illness, defined as hospital admission for clinical care rather than infection control. No deaths were reported. Compared with patients without HIV, patients with HIV were more likely to have diarrhoea (p=0·002), perianal rash or lesions (p=0·03), and a higher rash burden (median rash burden score 9 [IQR 6–21] for patients with HIV vs median rash burden score 6 [IQR 3–14] for patients without HIV; p<0·0001), but no differences were identified in the proportion of men who had severe illness by HIV status.
Interpretation
Clinical manifestations of monkeypox infection differed by HIV status. Recommendations should be expanded to include pre-exposure monkeypox vaccination of groups at high risk of infection who plan to engage in sexual or close intimate contact.
Funding
US Centers for Disease Control and Prevention, International Society of Travel Medicine.
Introduction
,
,
,
Two distinct clades, I and II, are known to cause disease, and clade II is associated with less severe disease.
,
The first monkeypox outbreak outside of Africa was reported in the USA in 2003 and was linked to contact with pet prairie dogs and other animals that were previously cohoused with infected small mammals imported from Ghana.
Travel-associated cases from Nigeria, some with secondary transmission, were also reported from Israel, Singapore, the UK, and the USA between 2018 and 2021.
,
,
,
,
,
,
,
,
and subsequently other regions,
,
prompting the WHO to declare the epidemic a Public Health Emergency of International Concern in July, 2022.
Local transmission has been sustained after virus introduction through international travel.
In contrast with previous reports of monkeypox, most but not all cases in the current epidemic have thus far been among gay, bisexual, or other men who have sex with men (MSM).
Infection is predominantly transmitted person to person through close, intimate contact with someone who has monkeypox.
,
Monkeypox virus transmission by sexual contact is not a novel concept,
,
,
,
but the predominance of such transmission in this epidemic is notable.
Evidence before this study
We searched PubMed for all articles related to monkeypox published between Jan 1, 1950, and August 3, 2022, using the search terms “monkeypox AND human”. Studies were included if they described clinical characteristics of human monkeypox cases. Studies were excluded if the transmission of all monkeypox cases in the report were animal-to-human transmission, with the exception of the US outbreak in 2013, which was notable due to its size and being the first outbreak outside Africa. Before the current monkeypox epidemic, only sporadic travel-associated monkeypox cases were reported, mostly among travellers who had been in Nigeria. Monkeypox virus transmission via sexual or close intimate contact has not been described before the 2022 epidemic, but was suspected to occur among cases in Nigeria, the UK, and Israel. There are few published reports about the epidemiology and clinical manifestations of patient cohorts with monkeypox in the context of the current multinational epidemic.
Added value of this study
We used surveillance data available from the GeoSentinel Network, which were the first clinical sites to identify monkeypox cases in the current epidemic, to investigate the epidemiological and clinical characteristics and outcomes of patients with monkeypox. We provide further evidence that transmission in the 2022 epidemic is predominantly (although not exclusively) person-to-person through close, sexual, or close intimate contact among men who have sex with men (MSM). Some patients met sexual partners at mass gatherings, and these events might contribute to the amplification of monkeypox virus transmission. This cohort included a large proportion of patients with HIV. No differences were identified in the proportion of patients with HIV who required treatment, admission to hospital, or were documented to have severe illness when compared with patients without HIV, which is likely to be due to their high CD4 counts, although patients with HIV were more likely to have diarrhoea and perianal rash or lesions, and have a higher rash burden. Rashes on the face, palm, and soles of feet, and lesions at the same stage of development and of the same size were not characteristic of the patients in this cohort.
Implications of all the available evidence
These data from GeoSentinel are notable for the predominance of sexual or close intimate contact as a mode of transmission, which was previously only suspected. Patients with HIV in this study were different from patients without HIV regarding clinical presentation, possibly due to deficient CD4 T-cell mediated immunity. Clinicians must maintain a high degree of suspicion for monkeypox and consider monkeypox when evaluating patients with rash and a history of sexual or close intimate contact. Health officials should consider working with organisations planning mass gathering events and community leaders to institute prevention measures, including advising attendees to not have sexual or close intimate contact with persons who have a rash and possibly expanding monkeypox vaccination recommendations to include pre-exposure monkeypox vaccination of groups at high risk of infection, such as MSM who plan to engage in sexual or close intimate contact. Framing monkeypox as a exclusively sexually transmitted infection is erroneous and potentially marginalising.
In this study, using data from confirmed cases of monkeypox infection, we aimed to describe epidemiological characteristics, routes of transmission, clinical findings, treatment courses, and outcomes, with subanalyses stratified by HIV and smallpox vaccination status, for individuals with monkeypox.
Methods
Study design and participants
We did a cross-sectional study using data obtained from patients included in GeoSentinel, a collaboration between the US Centers for Disease Control and Prevention (CDC) and the International Society of Travel Medicine, which is a global clinical-care-based surveillance system that monitors infectious diseases and other adverse health events that might impact international travellers and migrants. GeoSentinel comprises 71 clinical sites in 29 countries on six continents, where clinicians who specialise in travel and tropical medicine diagnose and treat patients and collect other relevant data. The protocol used by GeoSentinel for collection of surveillance data and the monkeypox questionnaire used have been reviewed by a human subjects advisor at CDC’s National Center for Emerging and Zoonotic Infectious Diseases and was determined to be public health surveillance and not human subjects research. All sites had ethical clearance for the collection of GeoSentinel surveillance data at their institution, which includes these data. Additional ethics clearance was obtained by some sites (McGill University Health Centre, Montreal, QC, Canada; Karolinska University, Stockholm, Sweden; and Francisco J Muñiz Infectious Disease Hospital, Buenos Aires, Argentina) as required by their respective institutions. Informed consent was not required by any of the institutions.
Procedures
Any cases of confirmed monkeypox infection clinically evaluated at GeoSentinel sites between May 1 and July 1, 2022 were eligible for inclusion. A supplemental data collection instrument for monkeypox-specific information was distributed to GeoSentinel sites and affiliates on May 22, 2022. In instances where the patient was seen before deployment of the questionnaire, patients were called via telephone to collect information not obtained during the initial patient examination. Some data for patients were missing, due to lack of patient willingness to respond or no documentation in the patient chart. Data collected included demographics, travel history including mass gathering attendance, smallpox vaccination history, social history, sexual history, monkeypox exposure history, medical history, clinical presentation, physical examination, testing results, treatment, and outcomes. For the physical exam, rash burden was indicated in an ordinal fashion (1 lesion, 2–10 lesions, 10–50 lesions, 50–100 lesions, >100 lesions); categories overlapped because the lesion count was estimated rather than counted.
A confirmed monkeypox case was defined as having a positive monkeypox virus PCR from skin lesions or serum, although additional specimens might have been collected and tested at the discretion of the treating clinician. MSM were defined as individuals who reported male sex assigned at birth and had sexual contact with at least one male partner in the 21 days before symptom onset. Close intimate contact was defined as cuddling, kissing, mutual masturbation, or sharing sex toys. A mass gathering was defined as an aggregation of more than 1000 people. A fever was defined as a temperature higher than 38°C. Severe illness was defined as hospital admission for clinical care (not for purposes of isolation unrelated to direct medical care) and was determined at the treating clinician’s discretion. Genital lesions were lesions located on the genitalia but not the anus (these were defined as perianal lesions). Samples were collected and cases managed according to the judgement of the individual clinicians.
Statistical analysis
Data were entered into a REDCap database (version 12.0.8) on a secured CDC server and analysed using R (version 4.1.1). The incubation period was estimated for patients with a known contact with a monkeypox case by calculating the time from the date of known contact to the date of symptom onset. The time to clinical presentation was estimated by counting days between symptom onset date and clinical visit date. To estimate overall rash burden, a composite lesion score was developed by summing the midpoints of the ranges for each anatomical location. For univariate analyses, we used χ2 (or Fishers exact tests when counts were <5) with a p value of less than 0·05 considered to indicate a statistically significant difference, to evaluate whether the proportion of patients with various clinical manifestations and outcomes was similar among patients with and without HIV, patients with HIV with CD4 counts of less than 500 cells per mm3 or 500 cells per mm3 and higher, patients with one sexual partner or ten or more sexual partners, and patients with or without a previous smallpox vaccination. We used non-parametric Wilcoxon rank-sum tests to assess difference among quantitative variables after assessing their distribution of values. Multivariable logistic regression models were used to better understand the relationship between HIV status and certain outcome variables. Multi-collinearity was assessed using the Condition Index (values of >15 indicate problems with multi-collinearity). Maximum likelihood χ2 p values were evaluated for significance (α=0·05).
Role of the funding source
The study funder collaborated with coinvestigators to design the questionnaire, and were involved in data analysis, data interpretation, and writing of the manuscript. The study funder had no involvement in data collection.
Results

Figure 1Map of countries where patients with confirmed monkeypox cases were reported at GeoSentinel sites, May 1–July 1, 2022 (n=226)

Figure 2Number of confirmed monkeypox cases reported to GeoSentinel by date of illness onset, May 1–July 1, 2022 (n=189)
Table 1Demographics, medical history, and travel information among confirmed monkeypox cases reported to GeoSentinel (May 1–July 1, 2022)
Data are n (%) or n/N (%), unless otherwise specified.
Table 2Exposure history among confirmed monkeypox cases reported to GeoSentinel (May 1–July 1, 2022)
Data are n/N (%), unless otherwise specified.
Table 3Clinical presentation, physical examination, and clinical course among confirmed monkeypox cases reported to GeoSentinel (May 1–July 1, 2022)
Data are n (%) or n/N (%), unless otherwise specified. STI=sexually transmitted infection.
23 (10%) of 226 patients received monkeypox treatment, most frequently with cidofovir (14 [6%]) or tecovirimat (ten [4%]). 30 (13%) of 226 patients were admitted to hospital; none required intensive care. Reasons for hospital admission were severe disease (16 [53%] of 30 patients), including severe pain (n=4), dysphagia with airway compromise (n=3), need for surgical drainage of lesions (n=1), Escherichia coli sepsis with high fever and with loss of consciousness (n=1), and unspecified (n=7). Other reasons for hospital admission included supportive care and medical curiosity. Ten (33%) of 30 patients were admitted to hospital for infection control purposes only. No patients died.
Table 4Comparison of clinical presentations, treatment, and outcomes among patients with confirmed monkeypox with HIV and without HIV reported to GeoSentinel (May 1–July 1, 2022)
STI=sexually transmitted infection.
Discussion
Nearly all patients enrolled in this study were MSM; almost half were individuals with HIV, most of whom were virally suppressed and had CD4 counts of 500 cells per mm3 or higher. All but three patients reported sexual or close intimate contact in the 21 days before symptom onset, and most reported multiple sexual partners, with a median of three partners. Fewer than 15% of patients required admission to hospital for medical care (severe illness). However, patients with HIV were more likely to have diarrhoea, perianal rash or lesions, and have a greater rash burden, but were not more likely to be treated or admitted to hospital than patients without HIV. Patients with CD4 counts lower than 500 cells per mm3 had a greater rash burden than patients with CD4 T-cell counts of 500 cells per mm3 or higher. Some patients met sexual partners at mass gatherings, possibly contributing to amplification of monkeypox virus transmission, and 16% had a concurrent STI. Patients most frequently presented with rash or skin lesions of various stages and sizes and almost two-thirds had lymphadenopathy, predominantly inguinal; fever was a common symptom, but almost two-thirds of individuals presented with rash.
This analysis had limitations. Not all individuals had a questionnaire initiated due to logistical issues. For individuals included in the analysis, information for some variables was not available for all patients because patients might have refused to answer or certain manifestations might not have been examined, tested, or recorded. Additionally, data were scarce with regard to where patients met sexual partners and in what circumstances exposures occurred, including if patients knew their partners had monkeypox at the time of sexual or intimate contact or learned about their partners’ diagnoses afterward. No data were collected on subsequent follow-up or long-term outcomes.
,
,
It remains unclear if fluids such as semen
,
and urine
can harbour viable monkeypox virus. Although transmission might occur directly from direct skin-to-skin contact with skin lesions, few patients reported having partners with an obvious rash. However, the monkeypox skin rash might be missed if lesions were in inconspicuous locations, such as the oral or anal mucosa.
Similarly, 75% of patients in this analysis had an incubation period of 11 days or less, with a median duration of 8 days. During the US outbreak in 2003, complex exposures (eg, invasive bite or scratch from an ill prairie dog) were associated with an incubation period of 9 days, suggesting that the route of infection might affect the incubation period.
One patient in our analysis had an estimated incubation period of 40 days, although this was based on self-report and should be interpreted with caution.
A high proportion of patients with HIV were included in our cohort. The presence of high HIV prevalence among some networks of MSM, behavioural aspects of patients with HIV that might increase the risk of STIs and monkeypox, or the nature of how cases are captured at GeoSentinel sites (which are travel and tropical medicine sites), might explain the high proportion. Since the median CD4 T-cell count among patients with HIV was high, the high proportion is unlikely to be due to increased monkeypox susceptibility from deficient T-cell-mediated immunity.
Patients with HIV had a higher rash burden than patients without HIV (especially among those with a CD4 count 3). Previous studies suggest that deficient CD4 T-cell immunity might have an effect on disease severity,
with prolonged illness, larger lesions, a greater number (>100) of lesions, and higher rates of secondary bacterial infections.
Public health officials should consider working with organisations planning mass gatherings and community leaders to institute prevention measures, including advising attendees to abstain from sexual or close intimate contact with people who have a rash to help curtail the spread of monkeypox.
Expansion of monkeypox vaccination recommendations
to include pre-exposure monkeypox vaccination of MSM who plan to engage in sexual or close intimate contact, including that associated with a mass gathering, are in concordance with WHO recommendations and can help limit person-to-person transmission.
,
Considering that fever and rash were the most common presenting symptoms in this cohort, monkeypox should be considered when evaluating patients with rash, and history of sexual or close intimate contact, even if fever is not present. However, the classic rash on the face, palm, and soles,
,
is not characteristic of the patients in this cohort, since genital lesions predominated. Also, more than half of patients had lesions in different stages of development or varying sizes, and more than 40% had lesions at different stages of development, which is also not characteristic of the characteristic monkeypox rash.
Absence of fever on physical exam was observed in many patients and should not rule out monkeypox. Considering the variation in findings on physical exam, obtaining a thorough exposure history for patients with suspected monkeypox, including testing for concurrent STIs, is important. Framing monkeypox as solely a STI is erroneous and can be stigmatising to groups such as MSM, while creating a false sense of security among others who are at risk.
Future studies could include research that focuses on the quantification and viability testing of monkeypox virus in various body fluids, even among asymptomatic individuals. The role of deficient T-cell immunity, if any, on disease severity should also be investigated via immunological studies.
Although GeoSentinel sites identified the first monkeypox cases in the current epidemic, these data might not be representative of the larger epidemic because patients needed to present to a GeoSentinel site or associated clinic to be included. However, by the nature of GeoSentinel sites’ clinical expertise in travel and tropical medicine, international travellers or patients who suspected their infection was monkeypox might have presented to a GeoSentinel site.
Contributors
KMA was involved in conceptualising the study, data curation, formal analysis, methodology, funding acquisition, project administration, resources, supervision, validation, visualisation, writing of the original draft, and review and editing the manuscript. TS was involved in conceptualising the study, data curation, formal analysis, methodology, resources, software, validation, visualisation, and writing, reviewing, and editing the manuscript. DC-F, LB-S, MDM, GS-N, SB, AD, KLBH, AC, EB, CG, MG, DMP, CPP, CM, CL, AdF, ES, MB, SL, CL, LB, HC, Ade, EF, SAF, HG, AG, MK, DM, AM, JM, DN, CR, PS, CT, and JVH were involved in data collection, resources, writing, and reviewing and editing the manuscript. HA was involved in conceptualising the study, data collection, data methodology, resources, and writing, reviewing, and editing the manuscript. SAJG was involved in conceptualising the study, data curation, formal analysis, methodology, resources, software, supervision, validation, visualisation, and writing, reviewing, and editing the manuscript. FW was involved in conceptualising the study, methodology, resources, and writing, reviewing, and editing the manuscript. RH, DHH. and PK were involved in conceptualising the study, methodology, funding acquisition, resources, visualisation, and writing, reviewing, and editing the manuscript. ML was involved in conceptualising the study investigation, methodology, funding acquisition, resources, supervision, visualisation, and writing, reviewing, and editing the manuscript. MG was involved with data visualization, and writing, reviewing, and editing the manuscript. LQ was involved in conceptualising the study, data curation, and writing, reviewing, and editing the manuscript. TS and SG directly assessed and verified data. All authors had full access to these data, read and approved the final version of the manuscript, and accept responsibility for publication.
Data sharing
Data collected for this analysis will not be made available to third parties due to the sensitive nature and data ownership rules with CDC and ISTM.
Declaration of interests
HA reports an unpaid leadership role as a member of the executive board of the International Society of Travel Medicine (ISTM) as counsellor and is a member of the boards of the Swedish Society of Tropical Medicine and of the Swedish Society of Travel Medicine. MD-M has received speaker fees from Pfizer, Gilead, and MSD unrelated to this project. SF has received speaker fees for Pfizer and MSD and serves on the advisory boards for MSD, Pfizer, AstraZeneca, and Gilead. AG reports grants from the The Netherlands Organisation for Health Research and Development for the COBRA–KAI study on COVID-19 vaccine immunogenicity in haematological patients; and has received fees for participation on the data safety monitoring board of the IDSCOVA-study on intradermal administration of COVID-19 vaccine. CG reports a grant from Gilead sciences in 2021 for a COVID-19 project; has received payment for meetings by AbbVie to develop hepatitis C educational materials for practitioners; and is also a member of the Committee to Advise on Travel and Topical Medicine. DH receives salary support from funding for GeoSentinel (1 U01CK000632-01-00) from the CDC’s Division of Global Migration and Quarantine, and he is also on the advisory board and collects speaking fees from Bavarian Nordic. RH and ML (principal investigator for GeoSentinel) receive salary support via the cooperative agreement between ISTM and the CDC for GeoSentinel (1 U01CK000632-01-00). MK reports consulting fees and institution grants from ViiV Healthcare, AbbVie, and Gilead for other unrelated projects. PK is vice president of the GeoSentinel Foundation (non-paid; voluntary). CP has received speaker fees from MSD and Pfizer; and is president of the Romanian Society for Travel Medicine and Infectious Diseases (unpaid). ES is a member of the executive board of the Asia-Pacific Travel Health Society; and is president of the Israeli Society of Parasitology and Tropical Medicine. JV reports travel expenses from the Flemish Research Foundation for participation in an international conference unrelated to this manuscript. All other authors declare no competing interests.
Acknowledgments
This project was funded through a Cooperative Agreement between the US Centers for Disease Control and Prevention (CDC) and the International Society of Travel Medicine (1 U01CK000632-01-00). The authors would like to thank the European Network for Tropical Medicine and Travel Health (TropNet) for their collegiality and collaboration. We would also like to thank Alba Antequera, (Barcelona, Spain; patient care), John T Brooks (Atlanta, GA, USA; manuscript review), Jaclyn Chabot (Montreal, QC, Canada; data entry), Maria Agustina Dal Molin (Barcelona, Spain; patient care), Cindy Friedman (Atlanta, GA, USA; protocol and manuscript review), Irene Fuertes De Vega, (Barcelona, Spain; patient care), Santiago Garro (Buenos Aires, Argentina; patient care), Miguel J Martinez (Barcelona, Spain; laboratory testing), Miguel Martinez-Lacalzada (Barcelona, Spain; patient care), Hansi Peiris (Montreal, QC, Canada; data entry), Mary G Reynolds (Atlanta, GA, USA; sample size calculations review), Aisha Rizwan (Atlanta, Georgia, USA; programme management), Natalia Rodriguez-Valero (Barcelona, Spain; patient care), Julie Rushmore (Atlanta, GA, USA; questionnaire development), and Lisa Owusu Sarpong (Montreal, QC, Canada; data entry). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US CDC.
Supplementary Material
References
- 1.
Monkeypox. Monkey signs and symptoms.
- 2.
Monkeypox. Monkeypox. Geneva: World Health Organization.
- 3.
Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo.
Viruses. 2017; 9: 283
- 4.
Monkeypox in 2022—what clinicians need to know.
JAMA. 2022; 328: 139-140
- 5.
Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003.
MMWR Morb Mortal Wkly Rep. 2003; 52: 642-646
- 6.
The changing epidemiology of human monkeypox—a potential threat? A systematic review.
PLoS Negl Trop Dis. 2022; 16e0010141
- 7.
Monkeypox transmission among international travellers-serious monkey business?.
J Travel Med. 2019; 26taz002
- 8.
Clinical features and management of human monkeypox: a retrospective observational study in the UK.
Lancet Infect Dis. 2022; 22: 1153-1162
- 9.
A case of imported Monkeypox in Singapore.
Lancet Infect Dis. 2019; 191166
- 10.
Monkeypox in a traveler returning from Nigeria—Dallas, Texas, July 2021.
MMWR Morb Mortal Wkly Rep. 2022; 71: 509-516
- 11.
Imported monkeypox from an international traveler, Maryland, USA, 2021.
Emerg Infect Dis. 2022; 28: 1002-1005
- 12.
Community transmission of monkeypox in the United Kingdom, April to May 2022.
Euro Surveill. 2022; 272200422
- 13.
Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022.
Euro Surveill. 2022; 272200424
- 14.
Epidemiological, clinical, and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022.
Euro Surveill. 2022; 272200421
- 15.
Coinfection of syphilis and monkeypox in HIV positive man in Prague, Czech Republic.
Travel Med Infect Dis. 2022; 49102368
- 16.
Monkeypox infection presenting as genital rash, Australia, May 2022.
Euro Surveill. 2022; 272200411
- 17.
Monkeypox outbreak—nine states, May 2022.
MMWR Morb Mortal Wkly Rep. 2022; 71: 764-769
- 18.
Second meeting of the International Health Regulations (2005) (IHR) Emergency Committee regarding the multi-country outbreak of monkeypox.
World Health Organization, GenevaJuly 23, 2022
- 19.
Joint ECDC-WHO Regional Office for Europe monkeypox surveillance bulletin.
- 20.
The 2017 human monkeypox outbreak in Nigeria—report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria.
PLoS One. 2019; 14e0214229
- 21.
Clinical course and outcome of human monkeypox in Nigeria.
Clin Infect Dis. 2020; 71: e210-e214
- 22.
Diagnosis of Imported Monkeypox, Israel, 2018.
Emerg Infect Dis. 2019; 25: 980-983
- 23.
Monkeypox virus infection in humans across 16 countries — April–June 2022.
N Engl J Med. 2022; 387: 679-691
- 24.
Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022.
Euro Surveill. 2022; 272200448
- 25.
Clinical manifestations of human monkeypox influenced by route of infection.
J Infect Dis. 2006; 194: 773-780
- 26.
HIV risk behaviors.
- 27.
Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations from the Advisory Committee on Immunization Practices—United States, 2022.
MMWR Morb Mortal Wkly Rep. 2022; 71: 734-742
- 28.
International outbreaks of Monkeypox virus infection with no established travel: a public health concern with significant knowledge gap.
Travel Med Infect Dis. 2022; 49102364
Article Info
Publication History
Published: October 07, 2022
Identification
Copyright
Published by Elsevier Ltd.
ScienceDirect
Linked Articles
- The 2022 monkeypox outbreak: the need for clinical curiosity
-
In the UK, the 2022 monkeypox outbreak was heralded by an imported case on May 7, 2022, with a typical history and presentation for imported monkeypox, including a widespread vesiculopustular rash and relevant travel history.1 Shortly afterwards, and perhaps due to public notification and increased awareness following this case, a cluster of cases was identified among gay, bisexual, and other men who have sex with men with no travel history and no epidemiological link to the index case.1 Since then, more than 60 000 laboratory confirmed cases have been identified in 105 countries and territories.
- Full-Text
-