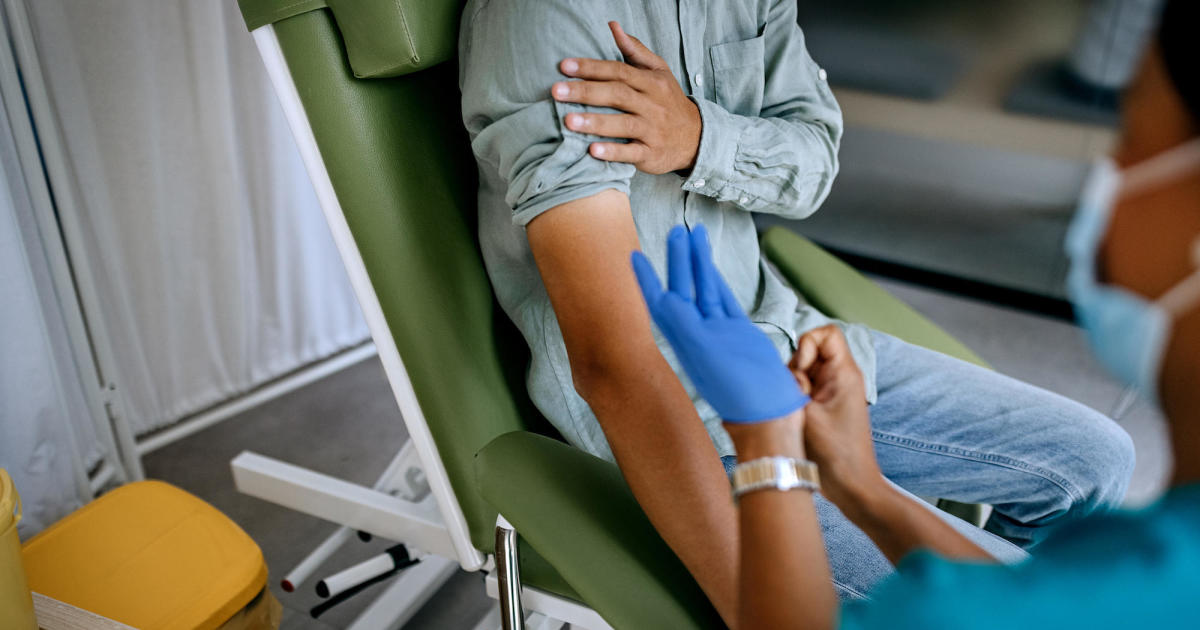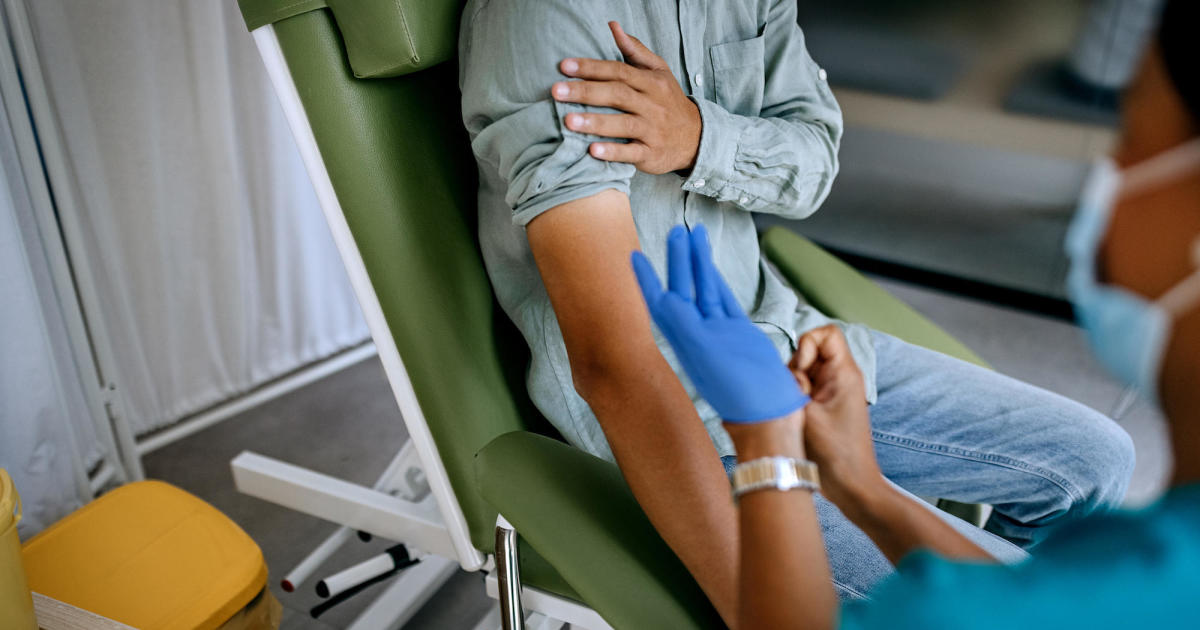
A panel of federal advisers could vote early next year on new recommendations about the nation’s blood supply, the Biden administration says, in one of the key final hurdles to ending a sweeping federal ban on blood donations from sexually active gay men.
The vote expected at the spring meeting of the Advisory Committee on Blood and Tissue Safety and Availability (ACBTSA) would come after the panel is able to review forthcoming data from the Food and Drug Administration’s ADVANCE Study.
The FDA announced last week that it now thinks the study’s results “will likely support” replacing the blanket ban, which currently requires gay men to abstain from sex for at least three months before donating blood. The Advance study has been looking at the effectiveness of using blood bank screening questionnaires to screen potential donors based on their individual risk factors, instead of broad “time-based deferrals.”
“This committee has played, and will continue to play an active role, in deferral policies. At the spring ACBTSA meeting next year, the committee will likely vote on a recommendation, after reviewing the data from the Advance Study,” a spokesperson for the Department of Health and Human Services tells CBS News.
An FDA spokesperson said Monday that the agency will consult the committee “as appropriate” as it works to “determine next steps.”
If the committee next year backs lifting the restrictions, the move would mark one of the last steps to undoing a controversial policy, first introduced in the 1980s and endorsed by the panel as recently as 2010, which had imposed a lifetime ban on gay men donating blood.
The lifetime ban was reduced in 2015 to allow donating blood after abstaining from sex for 12 months, and then down to three months in 2020.
While blood banks are now able to run tests to screen for HIV, there is still a risk that the virus could be missed during the “window period” early in a person’s infection.
“So now the Advance Study has finished enrolling, and everyone is very anxious to see the results, the analysis of the study,” says Dr. Claudia Cohn, director of the University of Minnesota’s Blood Bank Laboratory.
Cohn is also chief medical officer for the Association for the Advancement of Blood and Biotherapies (AABB), which represents the blood transfusion field, and chair of the ACBTSA.
“When it comes to data and blood safety, FDA is extremely and appropriately conservative. They’re not going to let anything change unless they have very, very high level of confidence that blood safety will not be negatively affected,” said Cohn.
A committee of AABB experts has been drafting a potential model for what the new risk-assessment questionnaires might look like, based on similar criteria that was adopted by Canada earlier this year.
A substantial revision to the AABB’s questionnaire is already under review by the FDA for approval, which addresses whether donors are taking medications, known as PrEP, to prevent HIV infection. That could interfere with the ability of blood banks to test donations for HIV.
The current Canadian questionnaire also includes questions about HIV PrEP drugs, as well as dozens of questions to gauge HIV risk, ranging from the number of partners to whether people have had sex with a sex worker.
Other questions in Canada’s screening include asking whether potential donors have recently had anal sex, and if they have had new or multiple sexual partners in the past few months.
“AABB has been working on this donor history questionnaire for a while, in preparation for this, but will not move on it until we know the results of the Advance Study and what the FDA wants to do,” Cohn said.
The FDA has yet to confirm exactly which questions were tried in its study. An AABB spokesperson also declined to comment on the questions in the study. The regulator says it looked at “similar risk factors that have been addressed by other countries,” but declined to reveal specifics.
“In an effort to maintain neutrality, objectivity and impartiality throughout the study, the questions used as part of the study remained confidential. Further information will be available once FDA’s analysis of the study results are complete,” an FDA spokesperson said.
It is also unclear how much ending the ban would do to improve the nation’s blood supply, which has faced shortfalls in recent years.
The American Red Cross in a statement said that it does “not have any data to show that a policy change would result in more blood donations.” In January, the organization had declared a dire first-ever “blood crisis” amid the worst shortage in over a decade.
That shortage has now been resolved, though the organization warned seasonal shortages could continue to occur without more people giving blood on a regular basis.
Preliminary findings from the National Blood Collection and Utilization Survey, presented at a November AABB meeting, suggests that blood collection “will keep pace with blood utilization requirements.”