Study design and participants
We conducted a prospective, observational, clinical feasibility study of ANNE at Pumwani Maternity Hospital (PMH) in Nairobi, Kenya, the largest referral public maternity hospital in sub-Saharan Africa. PMH has no neonatal intensive care unit. Caregivers of neonates delivered at or admitted to PMH were approached by trained study staff who obtained written informed consent and assessed the neonate for study eligibility based on the results of the medical history, clinical examination, and appropriate understanding of the study by the caregiver (Table 1). Carried out in accordance with the Declaration of Helsinki and Guideline for Good Clinical Practice/ International Standards Organization (ISO) 14155 to ensure accurate, reliable, and consistent data collection, the study protocol was approved by Western Institutional Review Board (20191102), Aga Khan University Nairobi Research Ethics Committee (2019/REC-02), and Kenya Pharmacy and Poisons Board (ECCT/19/05/02) and registered with ClinicalTrials.gov, NCT0392076110. Effort was made to ensure study participation did not interfere with or unnecessarily delay clinical care of neonates.
Study procedures
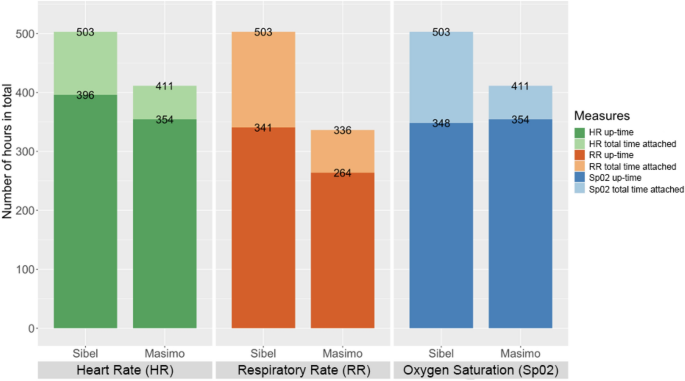
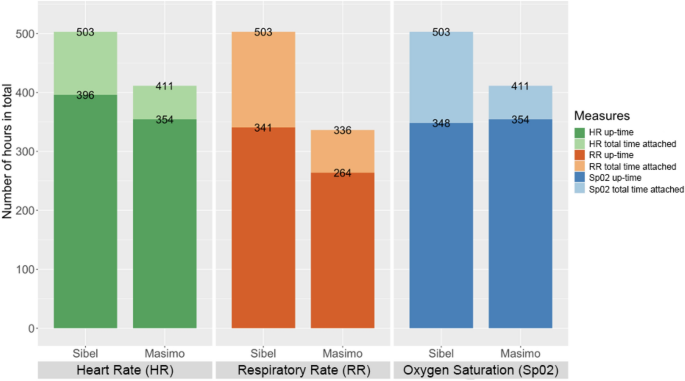
Enrolled neonates received local standard of care while additionally being simultaneously monitored with the ANNE multiparameter, continuous monitoring technology (Sibel Inc. IL, USA), the Rad-97 pulse CO-oximeter with capnography (Masimo Corporation, USA), and Spengler’s Tempo Easy non-contact infrared thermometer (SPENGLER HOLTEX Group, Aix-en-Provence, France). The Rad-97 was selected as a reference technology based on its ability to extract and record high resolution data, perform neonatal capnography and pulse oximetry, and its compact design enabling bedside monitoring. Tempo Easy was chosen as a reference technology for temperature monitoring due to its in-country availability. HR, RR, and SpO2 data were collected in real-time for a minimum of one hour from ANNE and Rad-97 and temperature data via hourly spot checks with the Tempo Easy technology. The total opportunity for attaching the technologies was similar. Up-time, clinical event detection performance, and HR, RR, SpO2, and temperature measurements data were collected by ANNE and the reference technologies (Table 1). For temperature, readings from ANNE’s chest sensor were compared to Tempo Easy’s skin temperature readings from the forehead.
Outcomes
To ensure objective measures of clinical feasibility, study outcomes included comparisons between the ANNE and reference technologies’ total time attached and up-time, and event detection of high and low HR and RR events, low SpO2 events, and high temperature events. In addition, we evaluated the agreement between HR, RR, SpO2, and temperature measurements in a real-world setting (Table 1).
Data processing and analysis
The total number of minutes the sensors were attached and the up-time for each technology were calculated. We assessed the quality of the measurements from the ANNE and reference technologies, and designated periods of adequate signal quality data as up-time (Table 1). For ANNE, we obtained HR, RR, and SpO2 measurements with their corresponding proprietary signal quality index every second. Raw data collected in real-time was retrieved from the Rad-97 with a custom Android application. Data were parsed in C (Dennis Ritchie & Bell Labs, USA) to obtain plethysmograph waveform and plethysmograph quality index (PO-SQI) data at 62.5 Hz (Hz), and capnography (carbon dioxide (CO2)) waveform data at approximately 20 Hz. CO2 waveform data were analyzed using a breath detection algorithm developed in MATLAB (Math Works, USA) based on adaptive pulse segmentation11. We obtained HR and RR from intra-beat and breath duration intervals, respectively. A custom algorithm based on capnography features was utilized to determine the capnography quality index (CO2-SQI). SpO2 values were calculated by the Rad-97 at 1 Hz, and 8-s medians were used in the analysis. To standardize event detection, upper limits for HR and RR were individualized for each neonate and were calculated for HR to be 20% and for RR to be 15% greater than their respective baseline values (based on a review of historical data) once the neonate was settled (approximately 15 min after monitoring was started) but no less than 140 beats/minute for HR and 40 breaths/minute for RR. For all neonates, an upper limit temperature of 37.5 °C was used. Lower limits were static with values of 80 beats/minute for HR, 15 breaths/minute for RR, and 90% for SpO2 based on the normal physiological range. To identify clinical events, Sibel provided a software parser that processed all of their monitoring data and provided event detection in pseudo- real-time that was finalized prior to data collection. Reference technology data were processed following data collection with a custom algorithm to identify events.
For overlapping periods of up-time from ANNE and Rad-97, clinical events were detected by examining the previous minute of data for both technologies. An event was identified if the one-minute median and the most recent ten-second median both met adequate signal quality and exceeded the threshold for upper or lower alarm values for HR, RR, SpO2, or temperature. Using a custom algorithm, events were aggregated from the technologies into ten-minute windows categorized as true positive, false negative, false positive, or true negative for high and low HR and RR and low SpO2 for each variable (Table 1)12. A panel of trained neonatologists and senior neonatology fellows conducted a manual adjudication of algorithmically-identified false negative and false positive events to identify those that were clinically significant (Table 1). For HR and RR, all false negative and false positive events that were greater than ten beats per minute for greater than two minutes were submitted for adjudication. For SpO2, all false negative and false positive events that had a difference greater than 4% between ANNE and Rad-97 for greater than two minutes were submitted for adjudication. Adjudication was not conducted for temperature monitoring due to a low number of events. Adjudication packages consisting of visualizations of selected false negative and false positive HR, RR, and SpO2 events and adjudication checklists were developed and provided to the panel of adjudicators (Supplementary Fig. S2). All false negative and false positive events were independently evaluated by two adjudicators and a third adjudicator reconciled the results if there was disagreement. To evaluate ANNE’s event detection, confusion matrices were generated and performance parameters including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and false negative-to-false positive ratio for high and low HR and RR, low SpO2, and high temperature were calculated for both pre- and post-adjudication results.
We evaluated measurement agreement between ANNE and the reference technologies as the normalized bias and the normalized spread between the 95% limits of agreement (LOA) calculated by dividing the bias and spread between the 95% LOA by the overall reference HR, RR, SpO2, or temperature mean value13. We also calculated the root-mean-square deviation (RMSD) for each comparison. Based on the reference technology verification phase, acceptable a priori-identified normalized spreads between the 95% upper and lower LOA of 30% were selected for both RR and HR14. For SpO2, the adequate a priori-identified limit for RMSD was 4.5% and the optimal limit was 3.5% based on standards from the International Organization for Standardization15. For temperature, an adequate a priori-identified limit of 4.5 °C for the LOA was selected based on a literature review and a formal validation study7. R version 4.0.2 (Vienna, Austria 2020) was used for the data analysis.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.