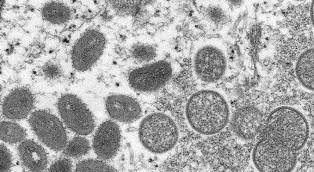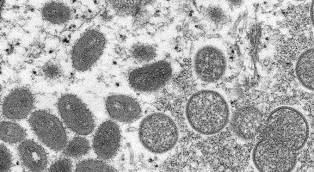
In recent months, the monkeypox incidence rates have greatly increased, prompting both the Biden Administration and the World Health Organization (WHO) to declare health emergencies for the virus. The former just declared in recent days, and allows for optimal mobilization of funding and resources to address the virus. The WHO did so back on July 23, saying it was a Public Health Emergency of the International Concern (PHEIC). At that time, monkeypox infections have been reported in 68 countries where the virus is not endemic. Worldwide, there were just under 17000 confirmed monkeypox infections.
Public Health Guidance
In its latest Morbidity and Mortality Weekly Report (MMWR), the Centers for Disease Control and Prevention (CDC) discussed prioritizing specific high-risk groups for monkeypox clinical care and education.
“Public health efforts should prioritize gay, bisexual, and other men who have sex with men, who are currently disproportionately affected, for prevention and testing, address equity, and minimize stigma, while maintaining vigilance for transmission in other populations,” MMWR reported. “Clinicians should test persons with rash consistent with monkeypox, regardless of whether the rash is disseminated or was preceded by prodrome.”
During the period of May 17-July 22, 2891 cases were reported in the United States including 43 states, Puerto Rico, and the District of Columbia. CDC received case report forms for 1,195 (41%) cases as of July 27.
Among the report forms, the confirmed cases in the United States occurred in 99% of men, and “94% of whom reported recent male-to-male sexual or close intimate contact.”
THE MMWR also pointed out racial and minority groups are being disproportionately affected.
“Among the 88% of cases with available data, 41% were among non-Hispanic White (White) persons, 28% among Hispanic or Latino (Hispanic) persons, and 26% among non-Hispanic Black or African American (Black) persons.”
And the presentations are atypical with fewer persons experiencing prodrome and more experiencing genital rashes.
Vaccines
The WHO has been involved in both smallpox and monkeypox health campaigns and reported in May the vaccines developed for the former also protect against the latter.
“Vaccines used during the smallpox eradication program also provided protection against monkeypox. Newer vaccines have been developed of which one has been approved for prevention of monkeypox,” WHO wrote.
WHO is recommending people in high-risk groups—including men who have sex with men and health care workers—get vaccinated.
In the US, states have had a limited vaccine supply. Vaccines are only being provided for people who are close contacts of those with confirmed cases.
And with the recent increase in demand, there may be some shortages in some areas in the US. For example, The San Francisco Department of Public Health (SFDPH) initially requested 35,000 doses of the monkeypox vaccine, and within the last week, San Francisco had only received approximately 12,000 doses.
Treatment
CDC and the Food and Drug Administration (FDA) have developed a process to help clinicians secure an antiviral therapy, tecovirimat (Tpoxx), for monkeypox treatment. This falls under the expanded access investigational new drug (EA-IND). Clinicians can begin the process to secure tecovirimat by going here.
Tecovirimat is manufactured by SIGA Technologies, and is approved for smallpox and is being studied for monkeypox treatment. Contagion spoke with SIGA Technologies Chief Scientific Officer Dennis Hruby, PhD, recently about the therapy.