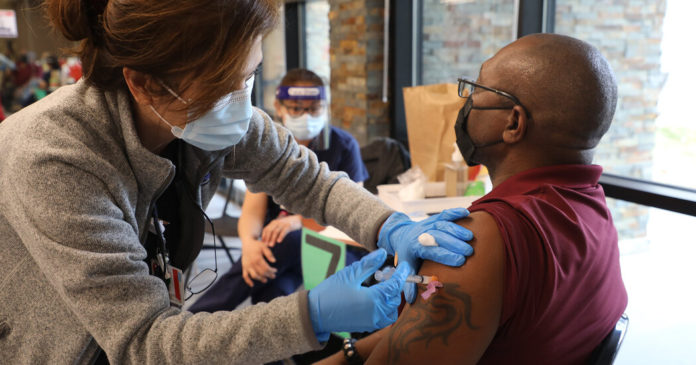
On Monday, Moderna said its vaccine is effective against new variants that have emerged in Britain and South Africa. But the immune response is slightly weaker against the variant discovered in South Africa, so the company is developing a new form of the vaccine that could be used as a booster shot against that virus.
And Dr. Rochelle Walensky, the new C.D.C. director, offered a blunt assessment of the vaccination campaign on Sunday, predicting that supply would not increase until late March.
Federal health officials and corporate executives agree that it will be impossible to increase the immediate supply of vaccines before April because of lack of manufacturing capacity. A third vaccine maker, Johnson & Johnson, is expected to report the results of its clinical trial soon. If approved, that vaccine would also help shore up production eventually, though the company has manufacturing issues of its own.
“I can’t tell you how much vaccine we have, and if I can’t tell it to you then I can’t tell it to the governors and I can’t tell it to the state health officials,” Dr. Walensky told “Fox News Sunday.”
What You Need to Know About the Johnson & Johnson Vaccine Pause in the U.S.
-
- On April 13, 2021, U.S. health agencies called for an immediate pause in the use of Johnson & Johnson’s single-dose Covid-19 vaccine after six recipients in the United States developed a rare disorder involving blood clots within one to three weeks of vaccination.
- All 50 states, Washington, D.C. and Puerto Rico temporarily halted or recommended providers pause the use of the vaccine. The U.S. military, federally run vaccination sites and a host of private companies, including CVS, Walgreens, Rite Aid, Walmart and Publix, also paused the injections.
- Fewer than one in a million Johnson & Johnson vaccinations are now under investigation. If there is indeed a risk of blood clots from the vaccine — which has yet to be determined — that risk is extremely low. The risk of getting Covid-19 in the United States is far higher.
- The pause could complicate the nation’s vaccination efforts at a time when many states are confronting a surge in new cases and seeking to address vaccine hesitancy.
- Johnson & Johnson has also decided to delay the rollout of its vaccine in Europe amid concerns over rare blood clots, dealing another blow to Europe’s inoculation push. South Africa, devastated by a more contagious virus variant that emerged there, suspended use of the vaccine as well. Australia announced it would not purchase any doses.
Mr. Biden’s travel ban is a presidential proclamation, not an executive order. Typically, proclamations govern the acts of individuals, while executive orders are directives to federal agencies. It will go into effect on Saturday and apply to non-U.S. citizens who have spent time in South Africa in the past 14 days. The new policy, which was reported earlier by Reuters, will not affect U.S. citizens or permanent residents, officials said.
The airline industry had no immediate comment on the new travel bans. But airline officials point to studies by the World Health Organization showing that the impact of travel bans on curbing the spread of infectious disease is limited. President Donald J. Trump came under intense criticism after he banned travel from China, and the ban proved porous as tens of thousands of people still came into the United States from that country.
“I think of travel bans as helpful but hardly foolproof,” said Dr. Ashish Jha, the dean of the Brown University School of Public Health. Mr. Trump’s China travel ban “probably slowed things down, and that’s reasonable,” he said. But travel bans need to be coupled with other policies to be useful, he said.