Fiber tension enables tissue scaling
Tissue development, homeostasis, and repair require cells to sense mechanical forces. Although many molecular actors implicated in cell mechanosensitivity have been extensively studied, the basis by which cells adapt their mechanical responses to their geometry remains poorly defined. López-Gay et al. now identify how two fundamental epithelial structures—stress fibers and tricellular junctions—endow Drosophila cells with an internal ruler to scale their mechanical response with their area. This work explains how cells of different sizes within an epithelial tissue collectively adapt their mechanical response to control tissue shape and proliferation. Scaling of biological properties with size is a core property of other biological systems.
Science, this issue p. eabb2169
Structured Abstract
INTRODUCTION
How biological properties scale with organ or body size is a question fundamental to development and physiology; however, at the cellular level, the scaling between size and properties such as mechanosensitivity remains poorly explored. Mechanosensitivity, the property by which cells sense mechanical forces, plays a fundamental role in proliferation and self-organization. In epithelial tissues, forces sensed at adherens junctions modulate cell behavior via the Hippo/YAP pathway. Although cell geometry, including apical cell area, can vary considerably among cells within a tissue, little attention has been given to whether or how epithelial cells scale their mechanical response to their size or whether such scaling is important for development.
RATIONALE
To probe the interplay between cell size and mechanical response, we first need to better understand how epithelial tissues respond to endogenous forces during morphogenesis. To characterize such tissue response via genetics, imaging, and mechanical perturbations, we used the Drosophila pupal dorsal thorax epithelium as a model system. Because, as in most tissues, cell size varies substantially within this epithelium, it is amenable to the investigation of the possible scaling of mechanosensitivity with cell size.
RESULTS
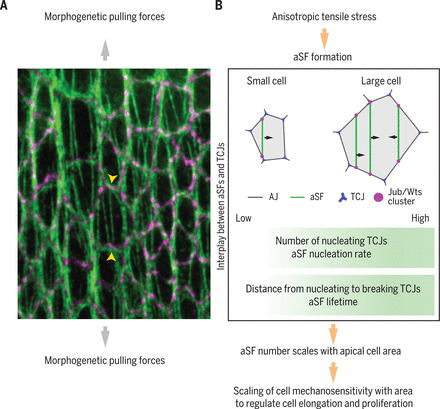
We observed that in response to morphogenetic forces, cells form apical stress fibers (aSFs), contractile actomyosin bundles that span the cell at the level of the adherens junctions. Through physical modeling and experiments, we found that the number of aSFs per cell scales with cell apical area. This scaling is critical to limit the elongation of larger cells relative to smaller cells under morphogenetic stress, and thus controls the final tissue shape. Moreover, because of the clustering of Hippo components at the tips of aSFs, the scaling of aSF number with cell apical area translates into a scaling between Hippo/YAP activation and cell area; the latter scaling favors the proliferation of larger cells and controls the final number of cells within the tissue.
To identify the “ruler” that enables the scaling of mechanosensitivity with cell area, we explored aSF dynamics. aSFs nucleate at tricellular junctions (TCJs), the position where three cells meet; aSFs then peel from the cortex and often break as they encounter another TCJ. Because both the number of TCJs and the separation between TCJs change as a function of cell area, we hypothesized that TCJs might provide an internal cell “ruler.” Predictions of computer simulations, experimentally tested via the modulation of TCJ number and positions, indicate that the scaling is mainly driven by the number of TCJs and their spatial distribution, which mediate an increase in aSF nucleation rate and lifetime in larger cells.
CONCLUSION
Our work uncovers a scaling between the number of aSFs per cell and cell apical area in response to morphogenetic stress. The number of TCJs and their spatial distribution largely account for this scaling. Thus, our work defines a functional link between TCJs and aSFs. Because TCJs and stress fibers are prevalent biological structures, the molecular characterization of their interplay might shed light on numerous aspects of tissue mechanics, proliferation, and morphogenesis.
(A) aSFs labeled by Myosin II (green) in the Drosophila pupal dorsal thorax epithelium under extensile morphogenetic stress (large gray arrows); the tips of one aSF are indicated by yellow arrowheads. Adherens junctions are labeled in purple by E-cadherin. (B) Schematic of the scaling of cell mechanosensitivity with cell area and the resulting control of tissue elongation and proliferation under anisotropic morphogenetic stress.
Abstract
Biological systems tailor their properties and behavior to their size throughout development and in numerous aspects of physiology. However, such size scaling remains poorly understood as it applies to cell mechanics and mechanosensing. By examining how the Drosophila pupal dorsal thorax epithelium responds to morphogenetic forces, we found that the number of apical stress fibers (aSFs) anchored to adherens junctions scales with cell apical area to limit larger cell elongation under mechanical stress. aSFs cluster Hippo pathway components, thereby scaling Hippo signaling and proliferation with area. This scaling is promoted by tricellular junctions mediating an increase in aSF nucleation rate and lifetime in larger cells. Development, homeostasis, and repair entail epithelial cell size changes driven by mechanical forces; our work highlights how, in turn, mechanosensitivity scales with cell size.